現在位置 : 藥物 > 帕金森病用藥 Madopar 美道普 125mg (低劑量) (L-DOPA)
|
商品名 : MADOPAR 125MG (低劑量)
中文名 : 美道普膠囊 健保局藥理類別 : 289200 其他中樞神經系統藥物 學名 : Levodopa 100mg, Benserazide 25mg 藥理作用 : Dopamine (DA)是腦中的神經傳遞物質,在帕金森氏病患的基底核沒有足夠的dopamine。 Levodopa (INN) 或 L-DOPA (3,4-dihydroxy L-phenylalanine) 為dopamine 生合成時之中間物質。Levodopa (dopamine 先驅物)為一前驅藥用來增加dopamine 濃度,因為它能穿過血-腦障壁,而dopamine 本身無法穿過血-腦障壁。一旦levodopa 進入中樞系統(CNS), 會被aromatic L-amino acid decarboxylase 代謝成dopamine。 給藥後,在大腦外和大腦組織中,levodopa 均會迅速去羧基成為dopamine,因此,大部分給予的levodopa 無法到達基底核,且在周邊系統產生的dopamine 會引起副作用。所以特別需要去抑制大腦外levodopa 的去羧基反應。同時給予levodopa 和周邊去羧基酶抑制劑 benserazide 可達到此效果。 適應症 : 治療帕金森氏病(Parkinson's Disease)。 用法用量 : 標準劑量:Madopar®治療以漸進方式給藥;應個別評估及調整劑量以達適當效果,因此以下的劑量用法應視為指導方針。 起始治療:建議開始治療早期帕金森氏症時,每天1 顆Madopar®‘62.5膠囊或半顆Madopar®‘125’錠劑3-4 次,一旦確認起始療程的耐受性後,再根據病人的反應慢慢增加劑量。 每日劑量相當於300-800 毫克的levodopa 和75-200 毫克的benserazide,分成3 次或更多次服用,通常可達到適當效果,可能需要4-6 星期方能達到適當的劑量。如有需要更進一步增加每日劑量,以每月調整一次為原則。 維持治療: 平均維持劑量是每天服用3-6 次 1 顆125 毫克的Madopar®膠囊或錠劑。必須調整服用次數(不小於3 次)和每天服用時間以達最適當效果。Madopar® HBS 可取代Madopar®一般劑型以達適當效果。 1.避免高蛋白飲食。2.初次使用可能頭暈。3.可能使尿液變成紅棕色。 藥動力學 : 吸收 : Levodopa主要在小腸的上半部吸收。投與 Madopar® 一般劑型,約1小時後可達到 levodopa 的最高血漿濃度。從 Madopar® 一般劑型中之levodopa的絕對生體可用率是 98% (範圍 74-112%)。Madopar® 一般劑型的膠囊和錠劑具生體相等性。 Levodopa的最高血漿濃度和吸收量(AUC)與劑量(50-200 mg levodopa)呈比例增加。 食物會降低 levodopa 的吸收速度和吸收量。在餐後給與一般劑型的Madopar®,levodopa 的最高血漿濃度會降低30%,且較慢出現,同時levodopa 的吸收量會降低15%。 分佈 : Levodopa 以可飽和的運輸系統穿透血-腦障礙,不會與血漿蛋白質結合且其分佈體積為57升。Levodopa在腦脊髓液的AUC是在血漿中的12%。 不同於levodopa,benserazide在治療劑量下不會穿透血-腦障礙,主要集中在腎臟、肺臟、小腸及肝臟。 代謝 : Levodopa經由兩個主要(去羧基化和鄰-甲基化)及兩個較次要的途徑(氨基轉移作用和氧化作用)代謝。 Levodopa利用芳香胺基酸去羧基酶轉換成dopamine,這個途徑的主要最終產物是homovanillic acid和dihydroxyphenylacetic acid。Levodopa利用catechol-O-methyltransferase甲基化變成3-O-methyldopa。這個主要血漿代謝物的排除半衰期為15小時,且會蓄積於接受Madopar®治療劑量的病人。 併用benserazide會降低周邊levodopa的去羧基化應,導致有較高血漿濃度的levodopa及3-O-methyldopa和較低血漿濃度的catecholamines (dopamine、noradrenaline)及phenolcarboxylic acids (homovanillic acid、dihydroxyphenylacetic acid)。 Benserazide在小腸黏膜和肝臟中會水解為trihydroxybenzylhydrazine,此代謝物是芳香胺基酸去羧基酶的強力抑制劑。 排除 : 在抑制周邊levodopa 去羧基酶的情形下,levodopa的排除半衰期約1.5小時,有巴金森氏病的老年患者(65-78歲)其排除半衰期較長(約25%),levodopa在血漿的清除率約430 ml/min。 Benserazide 幾乎完全利用代謝排除,其代謝物主要排泄於尿液(64%)及少量於糞便(24%)中。 副作用 : 有噁心、嘔吐和腹瀉。 交互作用 : 藥動學間的交互作用 併用抗乙醯膽鹼藥trihexyphenidyl 和Madopar®一般劑型,會降低levodopa 吸收速度但是對吸收量沒有影響。併用trihexyphenidyl 和Madopar® HBS 並不會影響levodopa 的藥動學。 併用制酸劑和Madopar® HBS 會降低levodopa 吸收量32%。 硫酸亞鐵降低levodopa 的最高血漿濃度和AUC 約30-50%。在與硫酸亞鐵併用治療時,所觀察到的藥動學變化似乎對某些病人有臨床上重要性,但不是對所有的病人。 Metoclopramide 會增加levodopa 的吸收速度。 Levodopa 和下列化合物沒有藥動學間的交互作用bromocriptine、amantadine、selegiline、和domperidone。 藥效學間的交互作用 安神劑、鴉片和含reserpine 的抗高血壓劑會抑制Madopar®的作用。 若對服用不可逆之非選擇性MAO 抑制劑的病人給予Madopar®時,在停用MAO 抑制劑和開始Madopar®治療間,至少須間隔2 星期,否則,副作用如高血壓可能發生選擇性MAO-B 抑制劑如selegiline 和rasagiline 和選擇性 MAO-A 抑制劑如moclobemide 可給予接受Madopar®治療的病人;建議依其療效和耐藥性,再調整levodopa 的劑量以配合每個病人的需要。併用MAO-A 和MAO-B 抑制劑的作用相當於非選擇性MAO 抑制劑,因此這種組合不應該再與Madopar®併用。Madopar® 不應併用擬交感神經藥物( 如會刺激交感神經系統的epinephrine 、norepinephrine、isoproterenol 或amphetamine),因為levodopa 可能加強這類藥物的作用,如果證實有併用的必要,必須緊密監測心血管系統,且擬交感神經藥物的劑量可能需要降低。 雖然療效及副作用均可能增強,仍允許併用其他藥物,如抗乙醯膽鹼藥、amantadine、多巴胺促動劑,惟可能需要降低Madopar®或其他藥物的劑量。當開始以COMT 抑制劑為輔助治療時,可能需要降低Madopar®的劑量。開始Madopar®治療時,不應該突然停用抗乙醯膽鹼藥,因為levodopa 需要一些時間才會開始有作用。Levodopa 可能影響catecholamines、肌酸酐、尿酸和葡萄糖的實驗室檢驗值。服用Madopar®的病人其Coombs’tests 的結果可能有偽陽性反應。 當此藥與含豐富蛋白質的食物共服時,其療效會降低。 禁忌 : 1.不能給予已知對levodopa 或benserazide 過敏的病人。 2.不可與非選擇性單胺氧化酶(MAO)抑制劑併用。 3.不能給與無足夠代償能力之內分泌、腎(洗腎病人除外)或肝功能異常,以及心臟功能異常、精神病、或閉角性青光眼的病人。 4.不能用於孕婦或在缺乏適當避孕下可能懷孕的婦女。 給付規定 : (光田綜合醫院) 1.3.4.帕金森氏症治療藥品:(91/11/1、93/2/1、95/9/1、96/9/1、97/7/1、100/6/1、101/6/1) 1.如病人開始出現功能障礙,在使用levodopa之前或同時,得使用一種dopamine agonist(ropinirole、pramipexole、pergolide、lisuride及rotigotine),或amantadine,或是levodopa併用 COMT抑制劑(entacapone:如Comtan film-coated tab.) 2.Levodopa+carbidopa+entacapone三合一製劑(如Stalevo Film-Coated Tablets 150/37.5/200mg等3品項): 限用於表現藥效終期運動功能波動現象,以左多巴/多巴脫羧基脢抑制劑無法達到穩定治療效果之巴金森氏症病人。(95/9/1) 3.若已同時使用上述藥物且達高劑量,仍無法達到滿意的 "on" state,或出現運動併發症(如異動症或肌強直),需合併使用多類藥物治療時,應於病歷上詳細記載理由。 4. Rasagiline:(101/6/1) (1) 可單獨使用,每日最高劑量為1 mg;或與levodopa併用,rasagiline每日最高劑量為0.5 mg。 (2) 本品不得與levodopa 以外之其他帕金森氏症治療藥品併用。 5. Pramipexole及ropinirole用於治療原發性腿部躁動症時需先排除腎衰竭、鐵缺乏症及多發性神經病變,且不得與dopamine agonist及levodopa併用。(96/9/1、97/7/1) (1) pramipexole每日最大劑量為0.75mg。(96/9/1) (2) ropinirole每日最大劑量為4mg。(97/7/1) 注意事項 : 1.不可突然停用,會導致體溫過高和肌肉僵直,心理上可能的改變,這些可能具生命威脅。 2.初期治療時,發生胃腸不良反應可併用食物,飲用水或慢慢增加劑量來控制。 3.尿液可能改變顏色,通常為紅色靜置後由淡轉暗。 過量處理 : 症狀和徵兆:過量可能會導致:心血管副作用(如心律不整)、精神障礙(如精神混亂和失眠)、胃腸道反應(如噁心和嘔吐)和異常不自主運動。 治療:監測病患的生命表徵及依病患臨床狀態著手進行支持療法。尤其是病患可能需要心血管作用(如抗心律不整劑)或中樞神經系統作用(如呼吸刺激劑、抗精神病劑)之症 狀治療。 藥品保存方式 : 藥品應置於攝氏 15 ~ 25 度乾燥處所;如發生變質或過期,不可再食用。 ========================================================== |
L-多巴(L-DOPA,全稱3,4-二羥苯丙氨酸)是酪氨酸經酪氨酸羥化酶的作用下羥化產生的一種氧化產物,具有兒茶酚羥基,可進一步生成另外一些有生物活性的物質:L-多巴在酪氨酸酶的作用下生成多巴醌繼而自發轉變為黑色素,或在芳香族胺基酸脫羧酶的作用下生成多巴胺,繼而形成去甲腎上腺素與腎上腺素等。
|
|
多巴胺(Dopamine)
多巴胺(dopamine)簡稱「DA」是一種腦內分泌物,屬於神經傳導物質(neurotransmitter),可影響一個人的情緒。因為它傳遞快樂、興奮情緒的功能,又被稱作快樂物質。阿爾維德·卡爾森確定多巴胺為腦內信息傳遞者的角色,這使他贏得了2000年諾貝爾醫學獎。 多巴胺是兒茶酚胺和苯乙胺家屬扮演在腦和身體的幾個重要作用的有機化學物。其名稱來自其化學結構:它是一個胺由其前體一個分子L-DOPA除去羧基合成,其發生在人腦細胞和腎上腺細胞中。在大腦中多巴胺作為神經傳導物質,通過神經元釋放一種化學物將信號發送到其它神經細胞。大腦包括幾個不同的多巴胺途徑,其中一個起著獎勵–激勵行為的主要作用。大多數類型的獎勵增加多巴胺在腦中的濃度,大部分成癮藥物增加多巴胺神經元活動。其他的腦多巴胺途徑參與運動控制和控制各種激素的釋放。 神經系統以外,在身體的幾個部分多巴胺(dopamine)作為局部化學信使的功能。在血管中它抑制去甲腎上腺素的釋放,並作為血管擴張劑(在正常濃度下);在腎臟中它增加鈉和尿的排泄;在胰臟中它減少胰島素生產;在消化系統中它減少胃腸蠕動和保護腸粘膜;並在免疫系統中它減少淋巴細胞的活性。血管除外,多巴胺在這些外圍系統局部合成,在鄰近該釋放它的細胞旁發揮其作用。 幾個重要的神經系統疾病與多巴胺系統的功能障礙有關,而使用幾個重要的神經系統疾病與多巴胺系統的功能障礙有關,而使用一些改變多巴胺作用的關鍵藥物來治療他們。帕金森氏病(PD)一種退行性狀況引起身體震顫和運動障礙,是通過中腦中稱為黑質區的分泌神經元分泌多巴胺不足所引起。其代謝前體L-DOPA可以工業製造,其純銷售形式為左旋多巴是最廣泛使用的治療方法。有證據表明精神分裂症涉及多巴胺活性水平的改變,大多數經常使用的治療抗精神病藥它具有降低的多巴胺活動的主要效果。類似多巴胺拮抗劑藥物,也有一些是最有效抗噁心藥物。不寧腿綜合徵(Restless leg syndrome)與注意力不足過動症與多巴胺活性降低有關。在高劑量多巴胺興奮劑可以上癮,但也有一些使用較低劑量治療過動症。多巴胺本身可製造成靜脈注射的藥物:雖然不能從血液到達腦部,其週邊作用使其對心臟衰竭或休克的治療是有用的,尤其是對新生嬰兒。 多巴胺簡介 多巴胺是一種用來幫助細胞傳送脈衝的化學物質,為神經傳導物質的一種。這種傳導物質主要負責大腦的情慾,感覺,將興奮及開心的信息傳遞,也與上癮有關。 愛情的感覺對應到生化層次,和腦裡產生大量多巴胺起的作用有關。 吸菸和吸毒都可以增加多巴胺的分泌,使上癮者感到開心及興奮。多巴胺傳遞開心、興奮情緒的這功能,醫學上被用來治療抑鬱症。 多巴胺不足或失調則會令人失去控制肌肉的能力、或是導致注意力無法集中。失去控制肌肉能力,在嚴重時會導致手腳不自主地顫動、乃至罹患帕金森氏症。 當我們積極做某事時,腦中會非常活躍的分泌出大量多巴胺。它是一種使人類引起慾望的大腦神經傳導物質,但多巴胺分泌過量會過度消耗體力和熱量,導致早死。 極端情形如亨丁頓舞蹈症,是多巴胺分泌過多而導致的疾病,患者的四肢和軀幹會如舞蹈般不由自主地抽動,造成日常行動不便,疾病發展到晚期,病人的生活將無法自理,失去行動能力,無法說話,容易噎到,甚至無法進食。 多巴胺最常被使用的形式為鹽酸鹽,為白色或類白色有光澤的結晶,無臭,味微苦。露置空氣中及遇光後色漸變深。在水中易溶,在無水乙醇中微溶,在氯仿或乙醚中極微溶解。熔點243℃-249℃(分解)。 多巴胺在人體的功能可分為神經系統內與神經系統外兩個部分。 多巴胺在腦的功能中,在運動控制、動機、喚醒、認知、獎勵的功能上扮演重要角色,還與一些更基礎的功能相關,例如哺乳、性慾、噁心。多巴胺類的神經元在人腦中的含量約有400,000個,其實是相對的少,並且有隻有在少數區域存在,但是卻投射到很多腦區,並能引起有很強大的功用。這些神經元最早在1964年由Annica Dahlström和Kjell Fuxe標繪出來,並給予這些區域A開頭的名字。在他們的模型中,A1-A7區包含正腎上腺素,A8-A14則包含多巴胺。以下是他們辨認出來包含多巴胺的區域: 黑質是中腦中一小塊形成基底核的區域,其中多巴胺神經元多在黑質的緻密部(A8)和其周遭(A9)被發現,和運動控制相關,若有失去大部分此區域的多巴胺神經元,會導致帕金森氏症。 腹側被蓋區(A10)則是另一塊屬於中腦的區域,是人腦中最多多巴胺神經元的地方,但實際上此區域仍然是非常的小。此區域的多巴胺神經元投射到伏核、前額葉皮質等其他區域,主要和獎勵、動機的功能相關。 下視丘後葉也有一些多巴胺神經元(A11),投射到脊髓,但功能並不是很清楚。 弓形核(A12)和腦室旁核(A14)都在下視丘,這些多巴胺神經元投射到腦垂腺前葉,透過中央聯合的循環組織,抑制催乳激素釋放細胞分泌催乳激素。通常說到這裡的調控時,多巴胺時常被稱為催乳素抑制因子、抑制催乳激素賀爾蒙、催乳激素抑制素。 一樣是在下視丘,不定區(A13)的多巴胺神經元則參與性腺激素釋放激素的控制。 還有多巴胺神經元位在視網膜,被稱為無軸突細胞,在日光的刺激下會活化,釋放多巴胺致細胞外基質中,相對的,在夜晚就會沈寂下來。這些視網膜中的多巴胺能夠抑制桿細胞而提升錐細胞的功能,最後產生對顏色敏感、並增加對比的效果,而其代價是在光線昏暗時便會降低其敏感度。 在神經系統外,在週邊,多巴胺也在侷限的區域透過外分泌或旁分泌產生功能: 首先是免疫系統,尤其是淋巴球,能夠製造並分泌多巴胺,其功能主要是抑制淋巴球的活性,但此系統的功能為何還並不是很清楚。 腎的小管細胞能分泌多巴胺,且腎有許多細胞能表現多種多巴胺受器,多巴胺在此能增加腎的灌流、提高腎絲球的過濾,並增加鈉離子的排泄。當腎部的多巴胺功能缺失時(可能肇因於高血壓或基因的問題),會導致鈉離子的排泄減少,造成高血壓。 胰臟也可以分泌多巴胺(外分泌),其功能可能與保護腸道的黏膜和降低嘗胃道蠕動相關,但還並不是很確定。 胰臟的胰島也和多巴胺相關,有證據顯示胰島的貝塔細胞製造胰島素時,也會製造多巴胺受器,這些受器受到多巴胺作用的結果是降低胰島素的釋放,但這些多巴胺的來源還沒有釐清的很清楚。 |
|
多巴胺結構
多巴胺(dopamine, DA)分子由兒茶酚結構(一個苯環與兩個羥基側基)經由乙基鏈連接一個胺基的。因此多巴胺可能是最簡單的兒茶酚胺類家族,包括神經傳導物質去甲腎上腺素和腎上腺素。存在一個苯環與胺連接,使得它取代的苯乙胺家族,其中包括大量的精神藥物。 像大多數胺,多巴胺是一種有機鹼。在酸性環境中,通常質子化。質子化形式是高度水溶性相對穩定的,但它是能夠被氧化,如果暴露於氧或其它氧化劑。在鹼性環境,多巴胺不會質子化。在這種游離鹼形式,它是更少水溶性,也比較反應性高。因為質子化形式增加穩定性和水溶性的,多巴胺提供化學或藥物使用的鹽酸多巴胺–即創建了鹽酸鹽, 當多巴胺與鹽酸結合。在乾燥形式時鹽酸多巴胺是一種精細無色粉末。 多巴胺生物化學 多巴胺是腦內極其重要的神經傳導物質(neurotransmitter),因為其作用特點又被稱作快樂物質。多巴胺屬於單胺類物質中的兒茶酚胺類,合成順序依次為酪氨酸-左旋多巴-多巴胺-去甲腎上腺素最後通過單胺氧化酶和兒茶酚胺氧位甲基移位酶酶解失活。合成腦內的3/4的DA細胞體位於中腦前部或者中腦。黒質包含了靈長類腦DA神經元的主要部分,黑質又可分為緻密部和網狀部。黑質DA神經元的主要投射部位尾核殼核伏隔核。大腦皮層是另一個主要投射部位。 多巴胺分類 目前共發現五種多巴胺受體,分為D1樣(D1 D5)D2樣(D2 D3 D4 )。DA受體都隸屬於G蛋白偶聯受體的超級家族。 多巴胺的釋放與降解 多巴胺(DA)的釋放是一種量子釋放,胞裂外排(exocytosis)。動作電位到達神經末梢時候,突觸前膜通透性發生改變,Ca離子進入細胞,促進囊泡附著於前膜,繼而形成小孔。由於嗜絡蛋白的收縮,將囊泡內容物排出。DA的降解分為兩類,一種是酶解,另一種是再攝取。DA及單胺類在神經末梢中再攝取占總排出量的四分之三,突觸間隙的DA可以被前膜,後膜,非神經組織攝取。先是通過細胞膜進入胞漿,這一階段由NA-K-ATP供能。第二步是囊泡攝取,這一步由Mg-ATP供能。酶解部分由單胺氧化酶和兒茶酚胺氧位甲基移位酶酶解失活。 主要多巴胺通道 ● 中腦皮層通路(mesocortical system) ● 中腦邊緣系統通道(mesolimbic system) ● 黑質紋狀體徑路(nigrostriatal system) ● 結節漏斗徑路(tuberoinfundibular system) 獎賞機制,多巴胺的獎賞通路,各種成癮物質均由位於中腦邊緣皮質的通路發生作用: 1.腹側被蓋核 2.伏隔核 3.前額葉皮層 作用於此通路,促進多巴胺的釋放使機體產生欣快感,停用後的戒斷反應等等。D1D2受體均參與自我給藥行為。 多巴胺與精神分裂症 引發精神醫學的革命性進展的藥物是氯丙嗪,它主要通過阻斷邊緣系統的D2受體發揮抗精神病作用。此後類似的藥物不斷被研發出來。 經典的精神分裂症的多巴胺假說:精神分裂症是由於多巴胺功能亢進造成的,一度在學術界占據壟斷地位,直到目前為止所有的精神分裂症假說都不能與多巴胺無關。 隨著第二代抗精神病藥物如氯氮平、利培酮的問世,其特點是對D2受體的低阻斷效果,更多的是對5-HT,NE受體的阻斷,調節谷氨酸多種受體發揮作用,對經典的多巴胺假說提出了質疑。 傳統的抗精神病藥物阻斷中腦邊緣系統D2受體發揮抗精神病作用,但是同時阻斷了黑質紋狀體的D2受體,引發錐體外系反應如肌張力上升,類帕金森症狀。泌乳素分泌增多,臨床上多採用苯海素,金剛烷胺,溴隱亭對抗以上不良反應。 歷史與發展 多巴胺最早是在1910年由喬治·巴格和詹姆斯·尤恩在英國倫敦惠康實驗室合成。於1957年凱瑟琳·蒙塔古首先在人的大腦中鑑定出多巴胺。它被命名為多巴胺,因為它是一種單胺,其前體是3,4-二羥基苯(左旋多巴胺)。在1958年阿爾維德·卡爾森在瑞典國家心臟研究所化學藥理學實驗室中最早認識到多巴胺作為神經傳導物質的功能。卡爾松被授予2000年諾貝爾生理學或醫學獎,其表明多巴胺不僅是去甲腎上腺素和腎上腺素的前體,而且自身也是神經傳導物質。 ============================================================ |
|
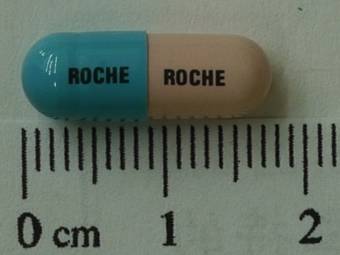
Madopar 125mg
商品名 : MADOPAR CAPSULES 125MG 中文名 : 美道普膠囊125公絲 主成份 : LEVODOPA, BENSERAZIDE HCL 100mg /25mg 劑型 : 膠囊劑 許可證字號 : 衛署藥製字第034888號 藥廠名稱 : 聯亞生技開發股份有限公司新竹廠 藥理治療分類 : 其他中樞神經系統藥物 孕婦等級 : D 外觀描述 : 長圓柱形 適應症 : 巴金森氏病。 用法用量: 1.起始治療:建議開始治療早期巴金森氏症時,每天1/2顆125公絲 3-4次。可增加至每日3-8顆125公絲膠囊 2.維持治療:平均維持劑量是每天服用3-6次1顆125公絲膠囊 3.在飯前至少30分鐘或飯後1小時服用,腸胃不良反應可能發生在治療初期,大部分可以併用食物、飲用水或慢慢增加劑量來控制。 副作用 : 1.厭食、噁心、嘔吐和腹瀉。 2.可能味覺喪失或改變。 3.心律不整或姿態性低血壓。 4. 少數曾發生溶血性貧血(hemolytic anemia)、短暫性白血球減少和血小板減少。 5.在治療較末期,不隨意運動可能發生。 (如舞蹈症(choreiform)或指痙病(athetotic)) 6. 老年患者可能發生情緒激動、焦慮、失眠、幻覺、幻想和暫時性方向感喪失。 7.肝臟轉氨酶(GOT、GPT)和鹼性磷酸酯酶(ALP)可能會短暫上升。 8.可能會使血中尿素氮(BUN)增加。 9. 尿液顏色可能會改變,通常為紅色,靜置後由淡轉成暗。 禁忌 : 1.內分泌無代償能力、腎或肝功能異常、心臟功能異常、有心理因素之精神病或閉角性青光眼的病人。 2.不能用於小於25歲的病人(骨骼發育必須健全) 3.不可與非選擇性MAOI一起服用。 (選擇性MAO-B抑制劑和選擇性 MAO-A 抑制劑可以使用) 4.不能用於孕婦或在缺乏適當避孕下可能懷孕的婦女。 注意事項 : 1. 不可以突然停用本品,可能導致NMS抗精神藥物惡性症候群(體溫過高和肌肉僵直、心理上可能的改變和serum creatinine phosphokinase的上升) 。 2. 對於有心肌梗塞,冠狀動脈循環不良或心律不整病例之病人,要週期性檢查心臟血管,包括心電圖。 3. 對患有廣角性青光眼之病人,應定期檢查眼內壓。 4. 當使用本品治療時不可突然中斷其他之帕金森氏症治療劑,因為前者療效之出現需要一段時間,所以只能慢慢減少。 5. 對於近期內患有胃潰瘍或骨質軟化症者要注意觀察。 6. 要經常注意血壓可以與抗高血壓劑併用。 7. 若因開刀而需全身麻醉,則必須事先停藥12-48小時。定期檢查血球數,肝臟及腎臟功能有其必要。 交互作用 : 1. 併用抗乙醯膽鹼藥trihexyphenidyl和本品,會降低levodopa 吸收速度但是對吸收量沒有影響。 2. Metoclopramide會增加levodopa的吸收速度。 3. 安神劑、鴉片和含reserpine的抗高血壓劑會抑制本品的作用。 4. 不應併用擬交感神經藥物(如會刺激交感神經系統的epinephrine、 norepinephrine、isoproterenol或amphetamine),因為levodopa可能加強這類藥物的作用,如果證實有併用的必要,必須緊密監測心血管系統,且擬交感神經藥物的劑量可能需要降低。 5. 當此藥與含豐富蛋白質的食物共服時,其療效會降低。 |
|
帕金森病(Parkinson's Disease, PD)
帕金森病西醫治療方法 罕見病發展中心 2014年03月20日 1. PD早期治療 帕金森氏病(PD)早期黑質-紋狀體系統存留的Dopamine(DA)神經元可代償地增加DA合成,推薦采用理療(按摩、水療)和體育療法(關節活動、步行、平衡及語言鍛煉、面部表情肌操練)等,爭取患者家屬配合,鼓勵患者多主動運動,盡量推遲藥物治療時間。若疾病影響患者日常生活和工作,需藥物治療。 2. 藥物治療 帕金森氏病(PD)目前仍以藥物治療為主,恢複紋狀體DA與Ach遞質系統平衡,應用抗膽堿能和改善DA遞質功能藥物,改善癥狀,不能阻止病情發展。 用藥原則: ①從小劑量開始,緩慢遞增,盡量用較小劑量取得滿意療效; ②治療方案個體化,根據患者年齡、癥狀類型和程度、就業情況、藥物價格和經濟承受能力等選擇藥物; ③不應盲目加用藥物,不宜突然停藥,需終生服用; ④帕金森氏病(PD)藥物治療複雜,近年來推出的輔助藥物DR激動藥、MAO-B抑制劑、兒茶酚-氧位-甲基轉移酶(COMT)等,與複方多巴合用可增強療效、減輕癥狀波動、降低複方多巴劑量,單獨使用療效不理想,應權衡利弊,適當選擇聯合用藥。 (1)抗膽堿能藥:對震顫和強直有效,對運動遲緩療效較差,適於震顫明顯年齡較輕患者。常用安坦(artane)1~2mg口服,3次/d;開馬君(kemadrin)2.5mg口服,3次/d,逐漸增至20~30mg/d。其他如苯甲托品(cogentin)、環戊丙醇(cycrimine)、安克痙(akineton)等,作用與安坦相似。副作用包括口幹、視物模糊、便秘和排尿困難,嚴重者有幻覺、妄想。青光眼及前列腺肥大患者禁用,可影響記憶功能,老年患者慎用。 (2)金剛烷胺(amantadine):促進DA在神經末梢釋放,阻止再攝取,並有抗膽堿能作用,是谷氨酸拮抗藥,可能有神經保護作用,可輕度改善少動、強直和震顫等,早期可單獨或與安坦合用。起始劑量50mg,2~3次/d,1周後增至100mg,2~3次/d,一般不超過300mg/d,老年人不超過200mg/d。藥效可維持數月至1年。副作用較少,如不安、意識模糊、下肢網狀青斑、踝部水腫和心律失常等,腎功能不全、癲癇、嚴重胃潰瘍和肝病患者慎用,哺乳期婦女禁用。也可用其衍生物鹽酸美金剛烷(memantine hydrochloride)。 (3)左旋多巴(L-dopa)及複方左旋多巴:L-dopa是治療帕金森氏病(PD)有效藥物或金指標。作為DA前體可透過血腦屏障,被腦DA能神經元攝取後脫羧變為DA,改善癥狀,對運動減少有特殊療效。由於95%以上的L-dopa在外周脫羧成為DA,僅約1%通過BBB進入腦內,為減少外周副作用,增強療效,多用L-dopa與外周多巴脫羧酶抑制劑(DCI)按4∶1制成的複方制劑(複方L-dopa),用量較L-dopa減少3/4。 複方L-dopa劑型:包括標準片、控釋片、水溶片等。標準片如美多巴(madopar)和帕金寧(sinemet):①Madopar由L-dopa與芐絲肼按4∶1組成,美多巴250為L-dopa 200mg+芐絲肼50mg,美多巴125為L-dopa100mg+芐絲肼25mg;國產多巴絲肼膠囊成分與美多巴相同;②帕金寧(Sinemet 250和Sinemet 125)由L-dopa與卡別多巴按4∶1組成。 控釋劑包括兩種: ①息寧控釋片(sinemet CR):L-dopa 200mg+卡別多巴50mg,制劑中加用單層分子基質結構,藥物不斷溶釋,達到緩釋效果,口服後120~150min達到血漿峰值濃度;片中間有刻痕,可分為半片服用,保持緩釋特性; ②美多巴液體動力平衡系統(Madopar-HBS):L-dopa 100mg+芐絲肼25mg及特殊賦形劑組成,膠囊溶解時藥物基質表面形成水化層,通過彌散作用逐漸釋放。 水溶片有彌散型美多巴(madopar dispersible),劑量為125mg,由L-dopa 100mg+芐絲肼25mg組成。其特點易在水中溶解,便於口服,吸收迅速,很快達到治療閾值濃度,使處於“關閉”狀態的PD患者在短時間內(10min左右)迅速改善癥狀,且作用維持時間與標準片基本相同。該劑型適用於有吞咽障礙或置鼻飼管、清晨運動不能、“開”期延遲、下午“關”期延長、劑末肌張力障礙的PD患者。 用藥時機:何時開始複方L-dopa治療尚有爭議,長期用藥會產生療效減退、癥狀波動及運動障礙等並發癥。一般應根據患者年齡、工作性質、疾病類型等決定用藥。年輕患者可適當推遲使用,早期盡量用其他抗PD藥,患者因職業要求不得不用L-dopa時應與其他藥物合用,減少複方L-dopa劑量。年老患者可早期選用L-dopa,因發生運動並發癥機會相對較少,對合並用藥耐受性差。 用藥方法:從小劑量開始,根據病情逐漸增量,用最低有效量維持。 ①標準片:複方L-dopa開始用62.5mg(1/4片),2~3次/d,根據需要逐漸增至125mg,3~4次/d;最大劑量不超過250mg,3~4次/d;空腹(餐前1h或餐後2h)用藥療效好; ②控釋片:優點是減少服藥次數,有效血藥濃度穩定,作用時間長,可控制癥狀波動;缺點是生物利用度較低,起效緩慢,標準片轉換成為控釋片時每天劑量應相應增加並提前服用;適於伴癥狀波動或早期輕癥患者; ③水溶片:易在水中溶解,吸收迅速,10min起效,作用維持時間與標準片相同,適於吞咽障礙、清晨運動不能、“開關”現象和劑末肌張力障礙患者。 副作用:周圍性副作用常見惡心、嘔吐、低血壓和心律失常(偶見)等,用藥後可逐漸適應,餐後服藥、加用嗎叮啉可減輕消化道癥狀。中樞性副作用包括癥狀波動、運動障礙和精神癥狀等,癥狀波動和運動障礙是常見的遠期並發癥,多在用藥4~5年後出現。閉角型青光眼、精神病患者禁用。 (4)DA受體激動藥:DA包括五種類型受體,D1R和D2R亞型與帕金森氏病(PD)治療關系密切。DR激動藥共同作用特點是:①直接刺激紋狀體突觸後DR,不依賴於DDC將L-dopa轉化為DA發揮效應;②血漿半衰期(較複方多巴)長;③可能對黑質DA能神經元有保護作用。早期DR激動藥與複方多巴合用,不僅能提高療效,減少複方多巴用量,且可減少或避免癥狀波動或運動障礙發生。 適應證:帕金森氏病(PD)後期患者用複方多巴治療產生癥狀波動或運動障礙,加用DR激動藥可減輕或消除癥狀,減少複方多巴用量。疾病後期因黑質紋狀體DA能系統缺乏DDC,不能把外源性L-dopa脫羧轉化為DA,用複方多巴完全無效,用DR激動藥可能有效。單用DA受體激動藥療效不佳,一般主張與複方L-dopa合用,發病年齡輕的早期患者可單獨應用。應從小劑量開始,漸增量至獲得滿意療效而不出現副作用。副作用與複方L-dopa相似,癥狀波動和運動障礙發生率低,體位性低血壓和精神癥狀發生率較高。 常用制劑:主要是溴隱亭、培高利特。 ①溴隱亭(bromocriptine):激活D2受體,開始0.625mg/d,每隔3~5天增加0.625mg,通常治療劑量7.5~15mg/d,分3次服;副作用與左旋多巴類似,錯覺和幻覺常見,精神病史患者禁用,相對禁忌證包括近期心肌梗死、嚴重周圍血管病和活動性消化性潰瘍等; ②培高利特(pergolide):激活D1和D2兩類受體,開始0.025mg/d,每隔5天增加0.025mg,一般有效劑量0.375~1.5mg/d,最大不超過2.0mg/d,1~3h達血漿峰值濃度,半衰期較長(平均30h),較溴隱亭抗PD作用稍強,作用時間亦長,溴隱亭治療無效時改用培高利特可能有效; ③泰舒達緩釋片(trastal SR):化學成分為吡貝地爾,是選擇性D2/D3多巴胺受體激動藥,劑量為150~250mg/d,對中腦-皮質和邊緣葉通路D3R有激動效應,改善震顫作用明顯,對強直和少動也有作用; ④麥角乙脲(lisuride):具有較強選擇性D2R激動作用,對D1R作用很弱,從小劑量開始,0.05~0.1mg/d,逐漸增量,平均有效劑量為2.4~4.8mg/d;按作用-劑量比,作用較溴隱亭強10~20倍,半衰期短(平均2.2h),作用時間短,為水溶性,可靜脈或皮下輸註泵應用,用於複方多巴治療出現明顯“開-關”現象; ⑤阿樸嗎啡(apomorphine):D1和D2R激動藥,可顯著減少“關期”狀態,對癥狀波動,尤其“開-關”現象和肌張力障礙有明顯療效,采取筆式註射法給藥後5~15min起效,有效作用時間60min,每次給藥0.5~2mg,每天可用多次,便攜式微泵皮下持續灌註法可使患者每天保持良好運動功能;也可經鼻腔給藥,但長期用藥可刺激鼻黏膜; ⑥卡麥角林(cabaser):是所有DR激動藥中半衰期最長(70h),作用時間最長,適於PD後期長期應用複方多巴產生癥狀波動和運動障礙患者,有效劑量2~10mg/d,平均4mg/d,只需1次/d,較方便; ⑦普拉克索(Pramipexole,0.125mg,3次/d,逐漸加量至0.5~1.0mg,3次/d)和羅吡尼洛(Ropinirole,0.25mg,3次/d,逐漸加量至2~4mg,3次/d),均非麥角衍生物,無麥角副作用,用於早期或進展期PD,癥狀波動和運動障礙發生率低,常見意識模糊、幻覺及直立性低血壓。 (5)單胺氧化酶B(MAO-B)抑制劑:抑制神經元內DA分解,增加腦內DA含量。合用複方L-dopa有協同作用,減少L-dopa約1/4用量,延緩開關現象,有神經保護作用。常用思吉寧(selegiline)即丙炔苯丙胺(deprenyl)2.5~5mg,2次/d,宜早、午服用,傍晚服用可引起失眠。副作用有口幹、胃納少和體位性低血壓等,胃潰瘍患者慎用。Lazabemide(Ro19-6327)亦系MAO-B抑制劑,目前臨床應用報道不多。 有學者主張此類藥與維生素E合用,稱DATA-TOP方案(deprenyl and tocopherol antioxidation therapy of Parkinsonism),作為神經保護劑用於早期輕癥患者,可能延緩疾病進展。維生素E是天然自由基清除劑,有抗氧化作用,PD早期尤其未經治療患者用維生素E與丙炔苯丙胺可能減緩黑質細胞變性、延緩疾病進展。近年國外有人提倡丙炔苯丙胺2.5mg口服,1次/d,漸加至2.5mg,2次/d,再加至5mg,2次/d;同時服用維生素E 2000U,1次/d。但目前對此方案仍有爭議,須繼續觀察評價。 (6)兒茶酚-氧位-甲基轉移酶(COMT)抑制劑:抑制L-dopa外周代謝,維持L-dopa穩定血漿濃度,加速通過BBB,阻止腦膠質細胞內DA降解,增加腦內DA含量。與美多巴或息寧合用增強後者療效,減少癥狀波動反應,單獨使用無效。副作用可有腹瀉、頭痛、多汗、口幹、轉氨酶升高、腹痛、尿色變淺等,用藥期間須監測肝功能。 常用制劑: ①托可朋(tolcapone):亦名答是美(tasmar),100~200mg口服,3次/d,副作用有腹瀉、意識模糊、運動障礙和轉氨酶升高等,應註意肝臟毒副作用;具有周圍和中樞COMT抑制作用,臨床試驗顯示,應用複方多巴療效減退的69例PD加用托可朋100~150mg,3次/d,療程6個月,有效率98.5%,無明顯毒副作用,可與複方多巴和MAO-B抑制劑合用; ②恩他卡朋(entacapone):亦名珂丹(comtan),是周圍COMT抑制劑,100~200mg口服,5次/d為宜,與托可朋不同的是迄今無嚴重肝功能損害報道。 (7)興奮性氨基酸(EAA)受體拮抗藥及釋放抑制劑:EAA可損害黑質細胞,抑制劑有神經保護作用,可增強L-dopa作用。但目前尚無臨床有效治療的報道。 (8)鐵螯合劑:PD患者黑質Fe2 濃度明顯增加,鐵蛋白含量顯著減少。給予鐵螯合劑可降低Fe2 濃度,減少氧化反應。目前常用21-氨基類固醇(21-aminosteroide),可通過血腦屏障與Fe2 結合,抑制脂質過氧化,對黑質細胞有保護效應。 (9)神經營養因子(neurotrophic factors):對神經元發育、分化及存活起重要作用,選擇性作用於DA能神經元的神經營養因子有助於PD防治。神經營養因子包括酸性及堿性成纖維細胞生長因子(aFGF、bFGF)、上皮生長因子(EGF)、睫狀神經營養因子(CNTF)、腦源性神經營養因子(BDNF)、膠質細胞源性神經營養因子(GDNF)及Neurturin等。GDNF和Neurturin對中腦DA能神經元特異性強。 (10)中藥或針灸對PD治療有一定的輔佐作用,需與西藥合用,單用療效不理想。 3.外科治療 立體定向手術治療帕金森氏病(PD)始於20世紀40年代,近年來隨著微電極引導定向技術的發展,利用微電極記錄和分析細胞放電特征,可精確定位引起震顫和肌強直的神經元,達到細胞功能定位,可顯著提高手術療效和安全性。手術可糾正基底節過高的抑制性輸出,適應證為藥物治療失效、不能耐受或出現運動障礙(異動癥)的患者,年齡較輕,癥狀以震顫、強直為主且偏於一側者效果較好,術後仍需用藥物治療。 (1)蒼白球毀損術(pallidotomy):近年來隨著微電極引導定向技術的發展,使定位精確度達到0.1mm,進入到細胞水平,達到準確功能定位,確定電極與蒼白球各結構及相鄰視束和內囊的關系,有助於尋找引起震顫和肌張力增高的神經元。用此法確定靶點,手術效果較好,改善PD運動癥狀,尤其運動遲緩,很少產生視覺受損等並發癥。 (2)丘腦毀損術:是用立體定向手術破壞一側丘腦腹外側核、豆狀襻及丘腦底核,對PD的震顫療效較好,最佳適應證是單側嚴重震顫。單側丘腦毀損術並發癥較少,雙側毀損術可引起言語障礙、吞咽困難及精神障礙等並發癥,不主張采用。 (3)深部腦刺激療法(deep brain stimulation,DBS):是將高頻微電極刺激裝置植入帕金森氏病(PD)患者手術靶點,高頻電刺激產生的電壓和頻率高於病變神經元產生的電壓和頻率,從而起到抑制作用。DBS優點是定位準確、損傷範圍小、並發癥少、安全性高和療效持久等,缺點是費用昂貴。美國FDA已批準臨床應用DBS治療PD。 (4)立體定向放射治療(γ-刀,X-刀):利用立體定向原理,用計算機精確計算靶點,一次大劑量窄束高能射線精確地聚焦破壞靶點,靶點外正常組織受劑量極小。射線包括60鈷(60CO)產生的γ-射線(γ-刀)及直線加速器產生的X射線(X-刀)。適應證與立體定向毀損術相同,但療效不如後者,副作用較多,目前不推薦使用。 4.細胞移植及基因治療 細胞移植是將自體腎上腺髓質或異體胚胎中腦黑質細胞移植到患者紋狀體,糾正DA遞質缺乏,改善帕金森氏病(PD)運動癥狀。酪氨酸羥化酶(TH)和神經營養因子基因治療正在探索中,是有前景的新療法。將外源TH基因通過exvivo或invivo途徑導入動物或患者腦內,導入的基因經轉錄、翻譯合成TH,促使形成DA。目前存在供體來源困難、遠期療效不肯定及免疫排斥等問題。 5.康復治療 對患者進行語言、進食、行走及各種日常生活訓練和指導,對改善生活質量十分重要。晚期臥床者應加強護理,減少並發癥發生。康複包括語音語調訓練,面肌鍛煉,手部、四肢及軀幹鍛練,鬆弛呼吸肌鍛煉,步態及平衡鍛練,姿勢恢複鍛練等。 預後: 帕金森氏病(PD)是慢性進展性疾病,目前無根治方法,多數患者發病數年仍能繼續工作,也可迅速發展致殘。疾病晚期可因嚴重肌強直和全身僵硬,終至臥床不起。死因常為肺炎、骨折等併發癥。 |
JJ
单击此处进行编辑.
|
|
L-DOPA
L-DOPA is a chemical that is made and used as part of the normal biology of humans, some animals and plants. Some animals and humans make it via biosynthesis from the amino acid L-tyrosine. L-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) collectively known as catecholamines. L-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Parcopa, Atamet, Stalevo, Madopar, and Prolopa. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia. L-DOPA has a counterpart with opposite chirality, D-DOPA. As is true with many molecules, the human body makes only one of these isomers (the L-DOPA form). Therapeutic Use L-DOPA crosses the protective blood–brain barrier, whereas dopamine itself cannot. Thus, L-DOPA is used to increase dopamine concentrations in the treatment of Parkinson's disease and dopamine-responsive dystonia. This treatment was made practical and proven clinically by George Cotzias and his coworkers, for which they won the 1969 Lasker Prize. Once L-DOPA has entered the central nervous system, it is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase. Pyridoxal phosphate (vitamin B6) is a required cofactor in this reaction, and may occasionally be administered along with L-DOPA, usually in the form of pyridoxine. Besides the central nervous system, L-DOPA is also converted into dopamine from within the peripheral nervous system. Excessive peripheral dopamine signaling causes many of the adverse side effects seen with sole L-DOPA administration. To bypass these effects, it is standard clinical practice to coadminister (with L-DOPA) a peripheral DOPA decarboxylase inhibitor (DDCI) such as carbidopa (medicines combining L-DOPA and carbidopa are branded as Lodosyn, Sinemet, Parcopa, Atamet, and Stalevo) or with a benserazide (combination medicines are branded Madopar or Prolopa), to prevent the peripheral synthesis of dopamine from L-DOPA. Coadministration of pyridoxine without a DDCI accelerates the peripheral decarboxylation of L-DOPA to such an extent that it negates the effects of L-DOPA administration, a phenomenon that historically caused great confusion. In addition, L-DOPA, co-administered with a peripheral DDCI, has been investigated as a potential treatment for restless leg syndrome. However, studies have demonstrated "no clear picture of reduced symptoms". The two types of response seen with administration of L-DOPA are: The short-duration response is related to the half-life of the drug. The longer-duration response depends on the accumulation of effects over at least two weeks, during which ΔFosB accumulates in nigrostriatal neurons. In the treatment of Parkinson's disease, this response is evident only in early therapy, as the inability of the brain to store dopamine is not yet a concern. |
Biological Role
L-DOPA is produced from the amino acid L-tyrosine by the enzyme tyrosine hydroxylase. It is also the precursor for the monoamine or catecholamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). Dopamine is formed by the decarboxylation of L-DOPA. L-DOPA can be directly metabolized by catechol-O-methyl transferase to 3-O-methyldopa, and then further to vanillactic acid. This metabolic pathway is nonexistent in the healthy body, but becomes important after peripheral L-DOPA administration in patients with Parkinson's disease or in the rare cases of patients with aromatic L-amino acid decarboxylase enzyme deficiency. L-Phenylalanine, L-tyrosine, and L-DOPA are all precursors to the biological pigment melanin. The enzyme tyrosinase catalyzes the oxidation of L-DOPA to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers. Side Effects The side effects of L-DOPA may include: ● Hypotension, especially if the dosage is too high ● Arrhythmias, although these are uncommon ● Nausea, which is often reduced by taking the drug with food, although protein interferes with drug absorption ● Gastrointestinal bleeding ● Disturbed respiration, which is not always harmful, and can actually benefit patients with upper airway obstruction ● Hair loss ● Disorientation and confusion ● Extreme emotional states, particularly anxiety, but also excessive libido ● Vivid dreams or insomnia ● Auditory or visual hallucinations ● Effects on learning; some evidence indicates it improves working memory, while impairing other complex functions ● Somnolence and narcolepsy ● A condition similar to stimulant psychosis Although many adverse effects are associated with L-DOPA, in particular psychiatric ones, it has fewer than other antiparkinsonian agents, such as anticholinergics and dopamine receptor agonists. More serious are the effects of chronic L-DOPA administration in the treatment of Parkinson's disease, which include: ● End-of-dose deterioration of function ● On/off oscillations ● Freezing during movement ● Dose failure (drug resistance) ● Dyskinesia at peak dose (levodopa-induced dyskinesia) ● Possible dopamine dysregulation: The long-term use of L-DOPA in Parkinson's disease has been linked to the so-called dopamine dysregulation syndrome. Clinicians try to avoid these side effects by limiting L-DOPA doses as much as possible until absolutely necessary. Possible Overdose Symptoms Some in vitro studies suggest a cytotoxic role in the promotion and occurrence of adverse effects associated with L-DOPA treatment. Though the drug is generally safe in humans, some researchers have reported an increase in cytotoxicity markers in rat pheochromocytoma PC12 cell lines treated with L-DOPA. Other authors have attributed the observed toxic effects of L-DOPA in neural dopamine cell lines to enhanced formation of quinones through increased auto-oxidation and subsequent cell death in mesencephalic cell cultures. There is no evidence of neurotoxicity in patients with Parkinson's disease and it is generally considered safe, but some controversy surrounds its use in the treatment of Parkinson's disease, given some test tube data indicate a deleterious effect on intracellular and neuronal tissue involved in the pathogenesis of the disease. |
|
Madopar (Roche)
Composition Active ingredients: levodopa and the decarboxylase inhibitor benserazide in the ratio of 4:1. The preparation is available as capsules of three different strengths and as cross‑scored tablets: Madopar ‘62.5’ (blue and light grey capsules) with 50 mg levodopa +12.5 mg benserazide (as hydrochloride); Madopar ‘125’ (blue and pink capsules) with 100 mg levodopa +25 mg benserazide (as hydrochloride); Madopar ‘250’ (blue and brown capsules) with 200 mg levodopa +50 mg benserazide (as hydrochloride); Madopar ‘125’ (pink tablets) with 100 mg levodopa +25 mg benserazide (as hydrochloride); Madopar ‘250’ (pink tablets) with 200 mg levodopa +50 mg benserazide (as hydrochloride). Properties Dopamine, which is not present in sufficient quantity in the basal ganglia of parkinsonian patients, acts as a neurotransmitter in the brain. Replacement therapy is achieved by administration of levodopa, the direct metabolic precursor of dopamine, since the latter substance has only a very limited ability to cross the blood‑brain barrier. After administration, however, levodopa is rapidly decarboxylated to dopamine, in cerebral as well as extracerebral regions. As a result, most of the levodopa administered is not available to the basal ganglia, and the dopamine produced peripherally frequently causes side effects. It is therefore particularly desirable to inhibit extracerebral decarboxylation of levodopa. This is achieved by simultaneous administration of levodopa and benserazide, a peripheral decarboxylase inhibitor. Madopar is a combination of these two substances in a ratio of 4:1 ‑ this ratio having proved optimal in clinical trials and therapeutic use ‑ and is thus just as effective as large doses of levodopa given alone while being much better tolerated. Combined administration of levodopa and benserazide thus makes it possible to compensate for dopamine deficiency in the brain. |
Pharmacokinetics Absorption Levodopa and benserazide are for the most part (66‑74%) absorbed in the upper regions of the small intestine. The maximum plasma concentration of levodopa is reached approximately one hour after ingestion of Madopar. Distribution The combined use of levodopa and benserazide compensates the dopamine deficiency in the brain. At therapeutic doses, benserazide does not penetrate the blood‑brain barrier. Metabolism Cerebral decarboxylase converts levodopa to dopamine, which in turn is converted‑to a minor degree‑to norepinephrine and‑to a greater part‑to inactive metabolites. Benserazide is hydroxylated into trihydrobenzylhydrazine in the intestinal mucosa and the liver. Elimination The elimination half‑life of levodopa is approximately 45 minutes. The elimination of levodopa and benserazide is principally via the kidneys. Indications Madopar is indicated for the treatment of all forms of Parkinson’s syndrome with the exception of druginduced parkinsonism. Contraindications Madopar must not be used in patients with known hypersensitivity to any of its ingredients. Patients should not be given monoamine oxidase inhibitors except selegiline while under treatment with Madopar. Madopar must not be given to patients with severely decompensated endocrine, renal, hepatic or cardiac disorders, psychoses or severe psychoneuroses. Madopar must not be given to patients less than 25 years old (skeletal development must be complete) or to pregnant women. Madopar is contraindicated in patients with narrow‑angle glaucoma. |
|
Side Effects
Anorexia, nausea and vomiting are rare when Madopar is used. Such side effects, which may occur in the early stages of treatment, can be largely brought under control by taking Madopar during meals or together with sufficient food or liquid, and by increasing the dosage slowly. Cardiovascular disorders (e.g. cardiac arrhythmias or orthostatic hypotension) may infrequently occur. At later stages of treatment, involuntary (e.g. choreiform or athetotic) movements may occur. These can usually be eliminated or be made tolerable by a reduction of dosage. A subsequent further stepping‑up of dosage in order to intensify the therapeutic effect may be attempted, since the side effects will not necessarily recur. Elevation of transaminase and alkaline phosphatase levels-though generally slight and not exceeding the upper limit of normal‑has occasionally been observed. Hemolytic anemia, as well as mild, transient leukopenia and thrombocytopenia have also been reported in a few rare cases. Therefore, as in any long‑term treatment, periodic blood counts should be made and liver and kidney function be tested. Mental disturbances (insomnia, agitation or, more rarely, depressive and-particularly in older patients‑psychotic reactions) may be observed. Precautions Intraocular pressure should be measured regularly during Madopar treatment in patients with wideangle glaucoma. Periodic cardiovascular checks (including ECG) should be performed in all patients with a history of myocardial infarction, coronary insufficiency or cardiac arrhythmia. Care should also be taken in patients with a history of gastric ulcer or osteomalacia. Other than in emergencies, Madopar therapy should be discontinued 1248 hours before surgical interventions requiring general anesthesia (see Drug Interactions). After surgery, medication with Madopar may be resumed, gradually increasing the dosage to the pre-operative level. If a patient must undergo surgery without Madopar having been withdrawn (e.g. in an emergency), anesthesia with cyclopropane or halothane should be avoided. Madopar should not be administered to patients with malignant melanoma (suspicious, undiagnosed lesions or evidence of melanoma in the history). Pregnancy and Lactation Animal studies have revealed fetotoxic effects, and no controlled trials have been conducted in man. The product must not be administered to pregnant women or nursing mothers. Women who become pregnant while being treated with Madopar must discontinue treatment immediately. Overdosage The most common symptoms of overdosage are involuntary movements, confusion, insomnia andmore rarely‑nausea, vomiting or cardiac arrhythmias. To treat overdosage, prompt evacuation of the stomach is recommended, as well as ECG monitoring of respiratory and heart function; it may be necessary to administer respiratory stimulants and/or antiarrhythmics, or, where appropriate, neuroleptics. Stability This medicine should not be used after the expiry date (EXP) shown on the pack. Drug Interactions Madopar may potentiate the effect of sympathomimetics given concomitantly. Close surveillance of the cardiovascular system is thus essential, and the dose of the sympathomimetic agents may need to be reduced. Because of the possibility of an additive effect when Madopar is used concurrently with antihypertensive agents, blood pressure must be regularly monitored in such cases. Neuroleptic drugs act as antagonists to the effects of Madopar. The effect of levodopa may be neutralized by vitamin B6. Such antagonism does not take place if levodopa is combined with a decarboxylase inhibitor. Therefore, Madopar may be administered at the same time as multivitamin preparations containing vitamin B6. Combination with other antiparkinsonian agents (anticholinergics, amantadine, dopamine agonists) is permissible, though this may intensify both the desired and the undesired effects. Dosage and Administration Standard Dosage Treatment with Madopar, as is usual for any levodopa therapy, should be introduced gradually; moreover, at all stages of the disease, dosage should be assessed individually and kept as low as possible. The following dosage instructions should therefore be regarded as guidelines. When taking Madopar capsules, patients must always ensure that they swallow the capsule without chewing it. Madopar tablets, however, may be broken down into small pieces to facilitate swallowing. Madopar should be taken during meals or with sufficient food or liquid. Initial Therapy In the early stages of Parkinson’s disease it is advisable to start treatment with 1 capsule of Madopar ‘62.5’ or ½ tablet of Madopar ‘125’ three or four times daily. Patients at a more advanced stage of the disease should receive twice as much. As soon as tolerability of the initial therapeutic schedule is confirmed, the dosage should be increased on a weekly basis by a single dose more per day (e.g. four daily doses instead of three, e.t.c.). If close supervision of the patient is possible, dosage adaptation may be made every two to three days. The optimal effect is generally reached at a daily dosage of 400‑800 mg levodopa +100‑200 mg benserazide, to be divided into three or more doses. If it proves necessary to further increase the daily dosage, this should be done on a monthly basis. Between four and six weeks may be needed to achieve the optimal dosage. Maintenance Therapy The average maintenance dosage is 1 capsule or 1 tablet of Madopar ‘125’ four to six times daily. The number of individual doses (not less than three) and their distribution throughout the day must be adapted to individual requirements. Special Dosage Instructions Non‑levodopa‑based antiparkinsonian agents can continue to be given until the full effects of Madopar are reached; after onset of the effect, however, they can often be gradually reduced. For patients who experience large fluctuations in the drug’s effect during the course of the day (on‑off phenomena) either more frequent and accordingly smaller single doses may need to be given. Dosage must be carefully titrated in every individual, including in elderly patients. |
|
Madopar
From NetDoctor How does it work? Madopar capsules and dispersible tablets contain the active ingredients levodopa and benserazide. This combination of medicines is also sometimes known as co-beneldopa. Co-beneldopa is also available without a brand name, ie as the generic medicine. It is used in Parkinson’s disease to increase the levels of dopamine in the brain. Dopamine is a substance known as a neurotransmitter. Neurotransmitters are found the brain and nervous system and are involved in transmitting messages between nerves. These messages help to perform certain functions the body. The neurotransmitter dopamine is known to be reduced or absent in the brains of people with Parkinson's disease, and this is thought to be the cause of the disease's symptoms. When you take levodopa, it is converted into dopamine in the brain. This replaces the lost dopamine and therefore reduces some of the symptoms of the disease. Levodopa is also converted into dopamine in the rest of the body, which can cause unwanted side effects such as nausea and palpitations. Benserazide is used in combination with the levodopa to prevent this happening. Benserazide is a type of medicine called a dopa-decarboxylase inhibitor. It blocks the conversion of levodopa to dopamine in the body and so prevents these side effects. (Benserazide cannot pass into the brain and so does not affect the conversion of levodopa to dopamine in the brain.) The combination of levodopa and benserazide is therefore effective in the treatment of Parkinson's disease, while minimising the side effects caused by levodopa on the rest of the body. Madopar dispersible tablets start to work more quickly than Madopar capsules. The tablets may be swallowed whole or dispersed in at least 25ml water per tablet. They may be taken in dilute orange squash (but not orange juice) if preferred. What is it used for? ● Parkinson's disease. Warning! ● This medicine can occasionally cause your blood pressure to drop when you move from a lying down or sitting position to sitting or standing, especially when you first start taking the medicine. This may make you feel dizzy or unsteady. To avoid this try getting up slowly. If you do feel dizzy, sit or lie down until the symptoms pass. ● This medicine can cause sleepiness and on rare occasions people have experienced a sudden onset of sleep during their daily activities. In some cases this can occur without any warning signs. Although this is rare, you should exercise caution when driving or performing other potentially hazardous activities. People who have experienced sleepiness or an episode of sudden onset of sleep while taking this medicine should not drive or operate machinery. Caution should be observed when drinking alcohol or taking other medicines that cause drowsiness, as this may increase the risk of drowsiness. ● As this medicine increases the level of dopamine in your brain more than levodopa alone it may cause abnormal involuntary movements or muscle twitches (dyskinesia). Consult your doctor if you experience these symptoms, as they may indicate that your dose of this medicine needs reducing. ● Consult your doctor if you feel depressed or confused, or have strange or abnormal thoughts while you are taking this medicine. ● Pathological gambling, increased sex drive and hypersexuality have been reported in people taking medicines for Parkinson's disease such as this one, which increase dopamine activity in the brain. If you think this medicine is affecting you in this way, you should consult your doctor. ● This medicine may cause a reddish discolouration of your urine and other body fluids, such as sweat and saliva. This is normal and not harmful. ● If your symptoms start to improve while taking this medicine, make sure you resume your normal activities gradually. Try not to do too much too quickly as you may risk injury. ● You should have regular tests to monitor the function of your liver, blood, kidneys and heart while taking this medicine. ● This medicine may affect the results of certain laboratory tests, including those for testing glucose levels in blood or urine. If you have diabetes ask your doctor for further information about this. Tell your doctor that you are taking this medicine if you have any blood or urine tests. ● If you have chronic open angle glaucoma you should have regular tests to monitor the pressure within your eye (intraocular pressure) while taking this medicine. ● You should not suddenly stop taking this medicine unless your doctor tells you otherwise. Use with caution in ● Liver disease. ● Kidney disease. ● Lung disease. ● Asthma. ● Disease involving the heart and blood vessels (cardiovascular disease). ● History of heart attack. ● History of irregular heart beats (arrhythmias). ● Disorders involving hormone producing glands (endocrine disorders). ● Diabetes. ● History of convulsions, eg epilepsy. ● History of peptic ulcers. ● History of psychiatric illness. ● Open angle glaucoma. ● Softening of the bones due to lack of vitamin D in the body (osteomalacia). Not to be used in ● People under 25 years of age. ● Severe liver disease. ● Severe kidney disease. ● Severe heart disease. ● Severe disease involving hormone producing glands (endocrine disease). ● Closed angle glaucoma. ● History of skin cancer (malignant melanoma). ● Suspicious skin lesions of unknown cause. ● Severe psychotic illness. ● Pregnancy. ● Breastfeeding. ● People who have taken a monoamine-oxidase inhibitor antidepressant (MAOI) in the last 14 days. This medicine should not be used if you are allergic to any of its ingredients. Please inform your doctor or pharmacist if you have previously experienced such an allergy. If you feel you have experienced an allergic reaction, stop using this medicine and inform your doctor or pharmacist immediately. Pregnancy and breastfeeding Certain medicines should not be used during pregnancy or breastfeeding. However, other medicines may be safely used in pregnancy or breastfeeding providing the benefits to the mother outweigh the risks to the unborn baby. Always inform your doctor if you are pregnant or planning a pregnancy, before using any medicine. ● This medicine should not be used during pregnancy as it may be harmful to an unborn baby. Women who could get pregnant should use effective contraception to prevent pregnancy while taking this medicine. Seek medical advice from your doctor. ● This medicine may pass into breast milk, therefore mothers taking this medicine must not breastfeed. Discuss with your doctor. Label warnings This medication may cause your urine to become a little red in colour. Side effects Medicines and their possible side effects can affect individual people in different ways. The following are some of the side effects that are known to be associated with this medicine. Just because a side effect is stated here, it does not mean that all people using this medicine will experience that or any side effect. ● Difficulty performing voluntary movements, resulting in jerky or involuntary movements or muscle twitches (dyskinesia). ● Disturbances of the gut such as diarrhoea, constipation, nausea, vomiting or abdominal pain. ● Mood changes, strange or abnormal thoughts or depression. ● Confusion. ● Awareness of your heartbeat (palpitations). ● Irregular heart beats. ● A drop in blood pressure that occurs when going from lying down to sitting or standing, which results in dizziness and lightheadedness (postural hypotension). ● Loss of appetite. ● Dry mouth. ● Weakness or loss of strength (asthenia). ● Difficulty in sleeping (insomnia). ● Sleepiness (somnolence). ● Suddenly falling asleep. ● False perceptions of things that are not really there (hallucinations). ● Bleeding or ulceration in the stomach or intestines. ● Disturbances in the normal numbers of blood cells in the blood. ● Inability to resist impulses to gambling (pathological gambling). ● Increased sex drive (libido) and excessive interest or involvement in sexual activity (hypersexuality). ● Visual disturbances. ● Difficulty in breathing (dyspnoea). The side effects listed above may not include all of the side effects reported by the medicine's manufacturer. For more information about any other possible risks associated with this medicine, please read the information provided with the medicine or consult your doctor or pharmacist. How can this medicine affect other medicines? It is important to tell your doctor or pharmacist what medicines you are already taking, including those bought without a prescription and herbal medicines, before you start treatment with this medicine. Similarly, check with your doctor or pharmacist before taking any new medicines while taking this one, to ensure that the combination is safe. This medicine should not be taken at the same time as, or within two weeks of taking non-selective monoamine oxidase inhibitors (MAOIs), for example the antidepressants phenelzine, isocarboxazid and tranylcypromine, and the antibiotic linezolid. In addition it should not be taken in combination with BOTH a selective monoamine oxidase type A inhibitor, eg moclobemide, AND a selective monoamine oxidase type B inhibitor, eg selegiline or rasagiline, though it can be used with either of these on their own. There may be an increased risk of dizziness when moving from a lying or sitting position to sitting or standing (postural hypotension) if this medicine is taken with other medicines that can have this effect, for example medicines to treat high blood pressure (antihypertensives). Your doctor may need to adjust the dose of your blood pressure medicine. Iron reduces the absorption of this medicine from the gut. For this reason, if you are taking iron supplements such as ferrous sulphate, you should take them two to three hours before or after this medicine. The following medicines may reduce the effect of levodopa: ● antipsychotic medicines, eg chlorpromazine, prochlorperazine, haloperidol, flupentixol (equally levodopa may oppose the effect of these medicines) ● benzodiazepines, eg diazepam ● isoniazid ● papaverine ● phenytoin. Tricyclic antidepressants, eg amitriptyline, imipramine, may decrease the therapeutic effects of this medicine, and may also rarely increase the risk of a dangerous rise in blood pressure (hypertensive crisis). Anticholinergic medicines, eg procyclidine, atropine, hyoscine, propantheline, orphenadrine, benzhexol, may decrease the absorption of this medicine from the gut and reduce its therapeutic effect. This medicine can be used with other medicines for Parkinson’s disease, eg anticholinergics, amantadine, dopamine agonists or COMT inhibitors, however, both the desired effects and side effects of treatment may be intensified. Your doctor may need to adjust the dose of one or more of your medicines to get the correct balance to control your symptoms with minimum side effects. |
|
Madopar Prolonged Release
4.2 Posology and method of administration Adults, including the elderly Dosage and administration are very variable and must be titrated to the needs of the individual patient. Madopar CR capsules must always be swallowed whole, preferably with a little water. They may be taken with or without food but antacid preparations should be avoided. In patients with nocturnal immobility, positive effects have been reported after gradually increasing the last evening dose to two Madopar CR 100 mg/25 mg capsules on retiring. Patients not currently treated with levodopa In patients with mild to moderate disease, the initial recommended dose is one capsule of Madopar CR three times daily with meals. Higher doses, in general, of Madopar CR will be required than with conventional levodopa-decarboxylase inhibitor combinations as a result of the reduced bioavailability. The initial dosages should not exceed 600 mg per day of levodopa. Some patients may require a supplementary dose of conventional Madopar, or Madopar Dispersible, together with the first morning dose of Madopar CR to compensate for the more gradual onset of the CR formulation. In cases of poor response to Madopar CR at total daily doses of Madopar CR plus any supplementary conventional Madopar corresponding to 1200 mg levodopa, administration of Madopar CR should be discontinued and alternative therapy considered. Patients currently treated with levodopa Madopar CR should be substituted for the standard levodopa-decarboxylase inhibitor preparation by one capsule Madopar CR 100 mg/25 mg per 100 mg levodopa. For example, where a patient previously received daily doses of 200 mg levodopa with a decarboxylase inhibitor, then therapy should be initiated with two capsules Madopar CR 100 mg/25 mg. Therapy should continue with the same frequency of doses as previously. With Madopar CR, on average, a 50% increase in daily levodopa dosage compared with previous therapy has been found to be appropriate. The dosage should be titrated every 2 to 3 days using dosage increments of Madopar CR 100 mg/25 mg capsules and a period of up to 4 weeks should be allowed for optimisation of dosage. Patients already on levodopa therapy should be informed that their condition may deteriorate initially until the optimal dosage regimen has been found. Close medical supervision of the patient is advisable during the initial period whilst adjusting the dosage. Children Not to be given to patients under 25 years of age: therefore, no dosage recommendations are made for the administration of Madopar CR to children. 4.3 Contraindications Madopar must not be given to patients with known hypersensitivity to levodopa or benserazide. Madopar must not be given in conjunction with non-selective monoamine oxidase (MAO) inhibitors. However, selective MAO-B inhibitors, such as selegiline and rasagiline or selective MAO-A inhibitors, such as moclobemide, are not contraindicated. Combination of MAO-A and MAO-B inhibitors is equivalent to non-selective MAO inhibition, and hence this combination should not be given concomitantly with Madopar (see section 4.5). Madopar must not be given to patients with decompensated endocrine (e.g. phaeochromocytoma, hyperthyroidism, Cushing syndrome), renal (except RLS patients on dialysis) or hepatic function, cardiac disorders (e.g. severe cardiac arrhythmias and cardiac failure), psychiatric diseases with a psychotic component or closed angle glaucoma. Madopar must not be given to patients less than 25 years old (skeletal development must be complete). Madopar must not be given to pregnant women or to women of childbearing potential in the absence of adequate contraception. If pregnancy occurs in a woman taking Madopar, the drug must be discontinued (as advised by the prescribing physician). Suspicion has arisen that levodopa may activate a malignant melanoma. Therefore, Madopar should not be used in persons who have a history of, or who may be suffering from, a malignant melanoma. 4.4 Special warnings and precautions for use When other drugs must be given in conjunction with Madopar, the patient should be carefully observed for unusual side-effects or potentiating effects. Hypersensitivity reactions may occur in susceptible individuals. Regular measurement of intraocular pressure is advisable in patients with open-angle glaucoma, as levodopa theoretically has the potential to raise intraocular pressure. Care should be taken when using Madopar in the following circumstances: in endocrine, renal, pulmonary or cardiovascular disease, particularly where there is a history of myocardial infarction or arrhythmia; psychiatric disturbances (e.g. depression); hepatic disorder; peptic ulcer; osteomalacia; where sympathomimetic drugs may be required (e.g. bronchial asthma), due to possible potentiation of the cardiovascular effects of levodopa; where antihypertensive drugs are being used, due to possible increased hypotensive action. Care should be exercised when Madopar is administered to patients with pre-existing coronary artery disorders, cardiac arrhythmias or cardiac failure (see also section 4.3). Cardiac function should be monitored with particular care in such patients during the period of treatment initiation and regularly thereafter throughout treatment. Close monitoring of patients with risk factors for (e.g. elderly patients, concomitant antihypertensives or other medication with orthostatic potential) or a history of orthostatic hypotension is recommended especially at the beginning of treatment or at dose increases. Madopar has been reported to induce decreases in blood count (e.g. haemolytic anaemia, thrombocytopenia and leukopenia). In a few instances agranulocytosis and pancytopenia have been reported in which the association with Madopar could neither be established, nor be completely ruled out. Therefore, periodical evaluation of blood count should be performed during treatment. Depression can be part of the clinical picture in patients with Parkinson's disease and RLS and may also occur in patients treated with Madopar. All patients should be carefully monitored for psychological changes and depression with or without suicidal ideation. Madopar may induce dopamine dysregulation syndrome resulting in excessive use of the product. A small subgroup of PD patients suffer from cognitive and behavioural disturbance that can be directly attributed to taking increasing quantities of medication against medical advice and well beyond the doses required to treat their motor disabilities. If a patient requires a general anaesthetic, the normal Madopar regimen should be continued as close to the surgery as possible, except in the case of halothane. In general anaesthesia with halothane Madopar should be discontinued 12 - 48 hours before surgical intervention as fluctuations in blood pressure and/or arrhythmias may occur in patients on Madopar therapy. Madopar therapy may be resumed following surgery; the dosage should be increased gradually to the preoperative level. If a patient has to undergo emergency surgery, when Madopar has not been withdrawn, anaesthesia with halothane should be avoided. Madopar must not be withdrawn abruptly. Abrupt withdrawal of the preparation may result in a neuroleptic malignant-like syndrome (hyperpyrexia and muscular rigidity, possibly psychological changes and elevated serum creatinine phosphokinase, additional signs in severe cases may include myoglobinuria, rhabdomyolysis – and acute renal failure) which may be life-threatening. Should a combination of such symptoms and signs occur, the patient should be kept under medical surveillance, if necessary, hospitalized and rapid and appropriate symptomatic treatment given. This may include resumption of Madopar therapy after an appropriate evaluation. Pyridoxine (vitamin B6) may be given with Madopar since the presence of a decarboxylase inhibitor protects against the peripheral levodopa transformation facilitated by pyridoxine. Levodopa has been associated with somnolence and episodes of sudden sleep onset. Sudden onset of sleep during daily activities, in some cases without awareness or warning signs, has been reported very rarely. Patients must be informed of this and advised to exercise caution while driving or operating machines during treatment with levodopa. Patients who have experienced somnolence and/or an episode of sudden sleep onset must refrain from driving or operating machines. Furthermore a reduction of dosage or termination of therapy may be considered (see section 4.7). Impulse control disorders
Patients should be regularly monitored for the development of impulse control disorders. Patients and carers should be made aware that behavioural symptoms of impulse control disorders including pathological gambling, increased libido, hypersexuality, compulsive spending or buying, binge eating and compulsive eating can occur in patients treated with dopamine agonists and/or other dopaminergic treatments containing levodopa, including Madopar. Review of treatment is recommended if such symptoms develop. Laboratory tests Periodical evaluation of hepatic, haemopoietic, renal and cardiovascular function and blood count should be performed during treatment. Patients who improve on Madopar therapy should be advised to resume normal activities gradually as rapid mobilisation may increase the risk of injury. Patients with diabetes should undergo frequent blood sugar tests and the dosage of antidiabetic agents should be adjusted to blood sugar levels. Malignant melanoma Epidemiological studies have shown that patients with Parkinson's disease have a higher risk of developing melanoma than the general population (approximately 2-6 fold higher). It is unclear whether the increased risk observed was due to Parkinson's disease, or other factors such as levodopa used to treat Parkinson's disease. Therefore patients and providers are advised to monitor for melanomas on a regular basis when using Madopar for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g. dermatologists). 4.5 Interaction with other medicinal products and other forms of interaction Pharmacokinetic interactions Co-administration of the anticholinergic drug trihexyphenidyl with standard dosage form of Madopar reduces the rate, but not the extent, of levodopa absorption. Trihexyphenidyl given concomitantly with Madopar CR formulation does not affect the pharmacokinetics of levodopa. Co-administration of antacids with Madopar CR formulation reduces the extent of levodopa absorption by 32%. Ferrous sulfate decreases the maximum plasma concentration and the AUC of levodopa by 30 – 50%. The pharmacokinetic changes observed during co-treatment with ferrous sulfate appeared to be clinically significant in some but not all patients. Opioids and drugs which interfere with central amine mechanisms, such as rauwolfia alkaloids (reserpine), tetrabenazine (Nitoman), metoclopramide, phenothiazines, thioxanthenes, butyrophenones, amphetamines and papaverine, should be avoided where possible. If, however, their administration is considered essential, extreme care should be exercised and a close watch kept for any signs of potentiation, antagonism or other interactions and for unusual side-effects. Metoclopramide increases the rate of levodopa absorption. Domperidone may increase the bioavailability of levodopa by stimulation of gastric emptying. Pharmacodynamic interactions Concomitant administration of antipsychotics with dopamine-receptor blocking properties, particularly D2-receptor antagonists might antagonize the antiparkinsonian effects of Madopar, therefore, should be carried out with caution, and the patient carefully observed for loss of antiparkinsonian effect and worsening of parkinsonian symptoms. Symptomatic orthostatic hypotension occurred when combinations of levodopa and a decarboxylase inhibitor were added to the treatment of patients already receiving antihypertensives. Madopar needs to be introduced cautiously in patients receiving antihypertensive medication. Blood pressure needs to be monitored to allow for potential dosage adjustment of either of the drugs, if required. Concomitant administration of Madopar with sympathomimetics (agents such as epinephrine, norepinephrine, isoproterenol or amphetamine which stimulate the sympathetic nervous system) may potentiate their effects, therefore these combinations are not recommended. Should concomitant administration prove necessary, close surveillance of the cardiovascular system is essential, and the dose of the sympathomimetic agents may need to be reduced. If Madopar is to be administered to patients receiving irreversible non-selective MAO inhibitors, an interval of at least 2 weeks should be allowed between cessation of the MAO inhibitor and the start of Madopar therapy. Otherwise unwanted effects such as hypertensive crises are likely to occur (see 4.3 Contraindications). Selective MAO-B inhibitors, such as selegiline and rasagiline and selective MAO-A inhibitors, such as moclobemide, can be prescribed to patients on levodopa-benserazide. It is recommended to readjust the levodopa dose to the individual patient's needs, in terms of both efficacy and tolerability. Combination of MAO-A and MAO-B inhibitors is equivalent to non-selective MAO inhibition, and hence this combination should not be given concomitantly with Madopar (see 4.3 Contraindications). Combination with other anti-Parkinsonian agents such as anticholinergics, amantadine, selegiline, bromocriptine and dopamine agonists are permissible, though both the desired and undesired effects of treatment may be intensified. It may be necessary to reduce the dosage of Madopar or the other substance. When initiating an adjuvant treatment with a COMT inhibitor, a reduction of the dosage of Madopar may be necessary. Anticholinergics should not be withdrawn abruptly when Madopar therapy is instituted, as levodopa does not begin to take effect for some time. Levodopa may affect the results of laboratory tests for catecholamines, ketone bodies, creatinine, uric acid and glucose. Levodopa therapy has been reported to inhibit the response to protirelin in tests of thyroid function. Coombs' tests may give a false-positive result in patients taking Madopar. A diminution of effect is observed when the drug is taken with a protein-rich meal. Concomitant administration of antipsychotics with dopamine-receptor blocking properties, particularly D2-receptor antagonists might antagonise the antiparkinsonian effects of levodopa-benserazide. Levodopa may reduce antipsychotic effects of these drugs. These drugs should be co-administered with caution. General anaesthesia with halothane: levodopa-benserazide should be discontinued 12-48 hours before surgical intervention requiring general anaesthesia with halothane as fluctuations in blood pressure and/or arrhythmias may occur. For general anesthesia with other anaesthetics see section 4.4. When Madopar CR is given with antacid preparations the bioavailability of levodopa is reduced, in comparison with conventional Madopar. 4.6 Use during pregnancy and lactation Madopar is contra-indicated in pregnancy and in women of childbearing potential in the absence of adequate contraception (see section 4.3 and section 5.3). Since it is not known whether benserazide passes into breast milk, mothers requiring Madopar treatment should not nurse their infants, since the occurrence of skeletal malformations in the infants cannot be excluded. |