現在位置 : RA > 類風濕性關節炎 - Rheumatoid Arthritis
|
疾病特徵
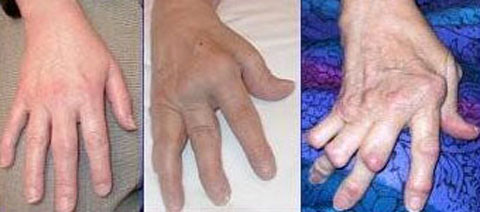
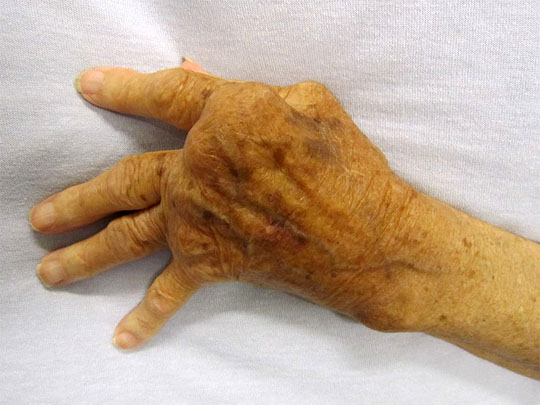
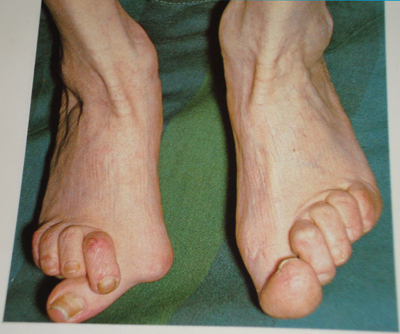
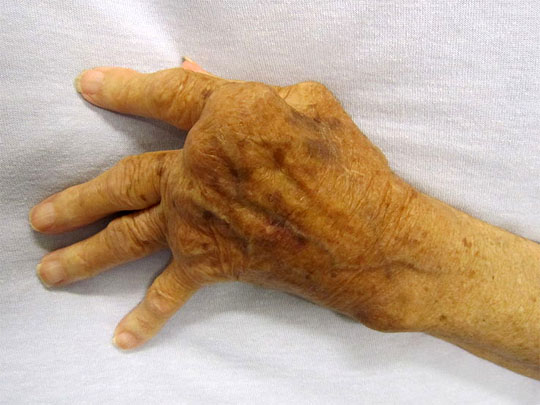
它的徵狀是多個不同的關節於同一時間發炎使到軟組織腫脹痛楚(多關節炎)。受影響的關節在開始時是不對稱的,在病勢發展時會逐步變為對稱。關節的痛楚會隨關節的運動而改善,在早上,關節會堅硬約一個小時。因此,類風濕性關節炎引致的痛楚在早上會較其他關節炎的痛楚厲害。 病勢會繼續發展至關節表面侵蝕及破壞,造成肢體畸形。手指一般會偏向小指(即尺側彎曲)及呈不自然的形狀。典型的類風濕性關節炎畸形是鈕釦畸形(即近端指骨間關節的過度屈曲及遠端指骨間關節的過度伸直)、鵝頸畸形(近端指骨間關節的過度伸直及遠端指骨間關節的過度屈曲)。拇指可能會發展成Z形拇指畸形,即掌指關節的固定性屈曲及關節移位,令手部形成正方形狀。 關節外的影響亦是另一個與骨關節炎不同的地方,所以類風濕性關節炎是一種多系統病症。就如大部份患有此症的病人都會同時患上貧血,這是因類風濕性關節炎本身的影響(慢性疾病引起的貧血症)或是因使用藥物(尤其是用作麻醉的非類固醇消炎止痛藥)治療時所有的腸胃道出血副作用。脾腫大亦會與白血球減少症一同出現(稱為費爾蒂綜合徵),及淋巴細胞浸入亦會影響唾液腺及淚腺(稱為乾燥綜合徵)。 以下是一些會受類風濕性關節炎影響的系統: 皮膚:在伸肌表面,如手肘出現類風濕結節。 肺部:肺部可能會直接受關節炎的影響,或是受治療的影響。治療後(如氨甲喋呤)可能會出現纖維症。 自身免疫:邊神經障礙、甲褶血管梗塞、神經病變及腎病。 腎臟:澱粉樣變性,亦會造成假性肌肉肥大。 心血管:心囊炎、瓣炎及纖維症。 眼睛:眼部乾燥症、鞏膜表層炎及鞏膜軟化症,亦會導致眼晴出現裂縫及滲漏。 神經:多數單神經炎及寰樞椎半脫位的徵狀。後者是因在頸椎與頭蓋骨的齒狀突或橫向韌帶的侵蝕,這種侵蝕(大於3毫米)會導致脊椎骨移位及壓逼脊椎。在開始時,病人會感到笨拙,若未能配合適切治療,會發展成四肢痲痺。 |
|
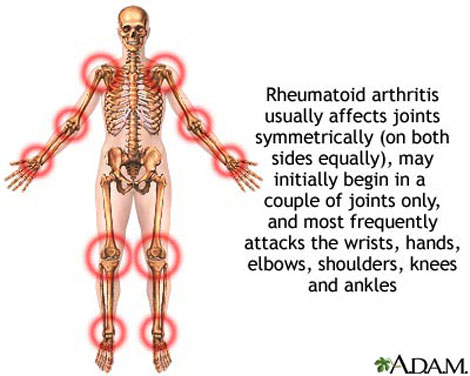
流行病學
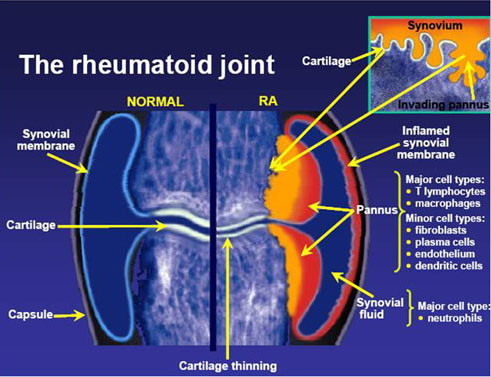
以35-50歲的人士患上類風濕性關節炎最多,家族遺傳會是一個重要的發病因素。它與人類白細胞抗原HLA-DR4有著關聯(W4、W14及W15與這病症有關,而W10及W13則是保護性的)。 類風濕性關節炎的發生率為每10,000的人口,就有30宗的個案。女性發病的機會是男性的3倍至5倍,而吸煙者發病的機會可以是非吸煙者的4倍。患病率約為1%,有些印第安人的患病率為5-6%,而加勒比海的黑人則有著較少的患病率。病人的一等親患病率為2-3%,而單卵雙胞胎的同患病率則是15-20%。 診斷標準 (Diagnosis) 美國風濕病學會於1987年定義以下的情況為類風濕性關節炎: ● 晨僵超過1小時; ● 3個或以上的關節發炎; ● 掌指、手腕和近端指間等關節出現關節炎; ● 對稱性的關節炎; ● 類風濕結節; ● 類風濕因子陽性; ● 放射線學(X光)檢測發現關節有侵蝕(erosion)。 只要達到最少任何以上四項情況,便被判斷為患有類風濕性關節炎。但在罹患此症的早期,某些上述的情況並不明顯,所以有些患者並非達至這一項標準。學會的目的是將病者分類作為研究,而非協助診斷此症。如其中一個情況是有關關節的磨損,在治療時這卻是主要要避免的情況,因關節磨損是不能治癒的。所以,縱然病人未能完全滿足上述的標準,亦會被當作患有此症而作出治療,以避免病情的惡化。雖然如此,這套標準仍然在研究方面非常有用,尤其在流行病學方面。 血液測試 (Blood Tests) 類風濕因子 (Rheumatoid Factor, RF) 當臨床測試懷疑患有類風濕性關節炎時,就要替病人作免疫學研究,如類風濕因子。類風濕因子呈陰性反應並不能完全撇開類風濕性關節炎的可能性,相反,可能會是一種稱為「血清反應陰性」的類風濕性關節炎。在患病的頭一年,類風濕因子測試多會是呈陰性的。其中80%的病人最終會轉為血清反應陰性狀態。在其他疾病,如乾燥綜合症,及約10%健康的人都會出現類風濕因子,所以測試並非十分準確。 抗環瓜氨酸抗體 (Anti-CCP) 由於類風濕因子測試不可靠,測試的專一性甚低,於是發展出新的血清測試:測試一種稱為抗含瓜胺酸蛋白質抗體的存在。這種測試能診斷出約80%的類風濕性關節炎病人,而在非患有類風濕性關節炎的病人中則很少呈陽性反應,專一性達至98%。這些抗體在早期病患時,甚至比發病前更早亦能測得。 為了測試其他關節炎的病因,亦會進行其他血液測試,如紅斑性狼瘡。此外,紅血球沉降速率(erythrocyte sedimentation rate, ESR)、C反應蛋白(C-reactive protein, CRP)、全血細胞計數、腎功能檢查、肝功能檢查及免疫檢查(如抗細胞核抗體,ANA)等亦會於此時進行。鐵蛋白能顯示模仿類風濕性關節炎的血色病。 病理生理學 類風濕性關節炎的成因仍未清楚,但一直都懷疑是因感染所致,亦可能是由食物敏感或外來生物所造成。支原體、詭譎丹毒絲菌、人類皰疹病毒第四型、B19細小病毒及風疹都是被懷疑而沒有佐證的流行病學研究。就如其他自身免疫疾病一樣,混淆身份理論指出一種入侵的生物引發免疫反應留下抗體,但這些抗體的獨特性卻不足夠,且開始攻擊與入侵生物相似的滑膜,這種現象稱"分子相似"。 患有自身免疫疾病的患者本身必須在分辨自身及外來分子的能力上有缺憾。在很多細胞內均有著自身辨認的標記。但是某一些標記卻容許類風濕性關節炎發生。約90%的病人都有著HLA-DR4/DR19標記,而對照中只有40%帶有此標記。因此在理論上,類風濕性關節炎的發生會受基因遺傳及一些感染事件來引發免疫反應。 一旦引發免疫反應,會使滑膜發炎。早期及中期發炎的分子媒介包括有"腫瘤壞死因子α" (TNF-α)、介白質-1、介白質-6、介白質-8及介白質-15、轉化生長因子β、纖維母細胞生長因子及血小板源生長因子。現代藥理學的治療都是針對以上的媒介。當炎症發生時,滑膜會變厚,軟骨與下面的骨頭開始碎裂及關節受到破壞。 除了以上外,生理及心理因素、壓力(stress)及不均衡的飲食在這病症中佔有一些地位 |
|
|
RA 治療
類風濕性關節炎的藥理學治療可以分為控制類風濕藥物、消炎藥及鎮病藥。控制類風濕藥物可以產生長久緩和、延後或停止病情的惡化,亦即阻止骨骼及關節由次級發炎至不能控制的損傷。消炎及鎮痛藥能減輕痛楚及改善僵硬,但卻不能阻止關節的傷害或減慢病情的惡化。 過往的策略是先使用消炎藥,臨床及使用X光檢查病情,如發現關節損壞的跡象,便使用控制類風濕藥物。現時很多病症在利用超聲波及磁力共振檢查後發現關節會在很早時期便受到損害。因此,若病人被診斷出類風濕性關節炎,便會及早使用控制類風濕藥物,以阻止進一步的關節損害。 早期使用控制類風濕藥物亦有另一個好處:在類風濕性關節炎發生早期,在關節內會充滿免疫細胞,它們互相傳信並發展出永久及慢性的發炎。利用控制類風濕藥物(如氨甲喋呤)可以阻礙這個步驟,並會改善日後關節炎的病情。 控制類風濕藥物 可再細分為異源生物體製劑及生物工程製劑。異源生物體製劑,與生物工程製劑相反,是一種並非在身體內自然產生的控制類風濕藥物。 異源生物體製劑 異源生物體製劑一般會造成一些副作用:如對肝臟、骨髓及腎臟的毒害、肺炎、皮膚敏感、自身免疫及感染等。 異源生物體製劑包括: ● 硫唑嘌呤 ● 環孢菌素 (即西克洛斯匹林A) ● 青黴胺 ● 氯金酸鈉 ● 氯奎寧 (Hydroxychloroquine (HCQ), Plaquenil) 氯奎寧雖然會引致眼睛的毒害(可是機會甚微),但由於它不會對肝臟及骨髓有所影響,故被認為是最低毒性的疾病改變抗風濕藥物。縱然如此,氯奎寧的藥性卻甚低,對於某些病人它根本不能控制病徵。 ● 來氟米特 ● 美諾四環素 ● 柳氮磺吡啶 ● 甲氨喋呤 -- 甲氨喋呤 (Methotrexate) 是抗代謝療法藥物,即是抗腫瘤藥,或預防移植體對宿主反應(排斥)之葯物。所以能夠壓抑骨髓活動及引致肝炎。同被視作毒性最高的藥物。甲氨喋(Methotrexate) 是目前最重要及有效的控制性抗風濕藥物。這是因為它能減輕部份白血球(抗體)發炎症活動,進一步阻慢骨骼的損害。 警惕事項:本藥僅可由對於抗代謝療法具有知識及經驗之醫師使用。高劑量治療惡性疾病曾有死亡之報告。 生物工程製劑 生物工程製劑包括: ● 腫瘤壞死因子α(TNF-α)抑制劑:依那西普(Enbrel®)、英利昔單抗(Remicade®)、復邁(Humira®) ● 白質1抑制劑:阿那白滯素 ● 抗CD20單抗:利妥昔單抗(Rituxan®) ● 白質6受體抑制劑:托珠單抗(Actemra®) 鎮痛及消炎藥 糖皮質激素 (類固醇藥物) ● 非類固醇消炎止痛藥(NSAID) ● 吲哚美辛(Indomethacin),是傳統的關節痛消炎藥;一天服用三次。此藥屬骨科止痛藥,份量應該由醫生決定。 副作用:常見有眩暈、惡心、頭痛、嗜睡和影響腸胃功能等。 ● Etoricoxib;ARCOXIA為一非類固醇消炎止痛藥(NSAID),可選擇性地抑制環氧酶-2(COX-2),以阻斷前列腺素合成,能舒緩痛楚及炎症。是默克製藥有限公司的用於關節炎和疼痛的研究性藥物,具有抗發炎、鎮痛及解熱的作用。每天120 mg Arcoxia (etoricoxib)緩解病人疼痛。效果不異於服用吲哚美辛 (50 mg,一天三次)。如非必要,不應長時間服用Etoricoxib。此藥屬骨科止痛藥,份量應該由醫生決定。 副作用:常見有眩暈、惡心、頭痛、嗜睡和增高血壓等。胃腸毒性較低,也不會影響血小板的功能。Arcoxia目前在許多疾病上都在進行研究,包括骨關節炎、類風濕性關節炎、急慢性疼痛、痛經、急性痛風濕性關節炎和強直性脊柱炎等。 其他止痛藥包括有: ● 對乙醯氨基酚 ● 鴉片劑 ● 利多卡因 研究 減輕痛楚 最近的研究指出細胞因子(即一組由身體不同細胞生產的化合物)可能是產生類風濕性關節炎的慢性痛楚的主因。使用藥物以影響細胞因子的釋放或阻礙細胞因子的運作能減輕慢性痛楚。不同種類的抗細胞因子藥物現已被使用作治療類風濕性關節炎或克隆氏症等痛症。另外,現時有研究使用沙利度胺來治療因脊椎蜘蛛膜炎所產生的痛症。 其他治療 其他治療有減肥、職能治療、物理治療、關節注射及一些特別的工具改善運動。嚴重受傷的關節則需要進行人工關節置換術,如置換膝部關節。 |
過敏免疫風濕科 常用藥物介紹
以下內容為簡介, 如有任何問題, 請再查詢或詢問醫師或藥師 免疫抑制劑:
(一) Methotrexate (MTX) 2.5mg (Trexan/治善錠):此藥用法特殊,一般每週劑量為7.5mg;可能一次服下,也可能以12小時或一天為間隔分成三次使用。副作用多為血球方面的問題,應定期的監測血球及肝腎功能。 (二) Azathioprine 50mg (Imuran/移護寧錠):因免疫抑制較強,肝毒性也較大,一般作為其他藥物無法控制疾病時使用。使用者須密切監測骨髓抑制現象,必要時停藥,以避免感染。(三) Leflunomide 10mg(Arava/艾炎寧膜衣錠):為新型藥物,一開始必須連續三天使用高劑量,之後維持每日20mg,約一個月開始出現治療效果。特別注意,使用以上藥品皆需進行避孕,如果考慮懷孕,應事先與醫師進行討論。停藥後並非立即可以懷孕,須有等待期並檢測留在體內的藥品是否排除完全,以確保胎兒安全。 免疫調節劑:
(一) Sulfasalazin (SSZ) 500mg (Salazopyrirc EN/斯樂腸溶錠):一天1000mg分兩次隨餐使用,如醫師評估需要,可增加至一日2000mg。副作用為皮疹、血球減少、頭痛發燒 。一開始需較密集地血球監測。蠶豆症的人不可使用。 (二) Hydroxychloroquine (HCQ) 200mg (Plaquenil/必賴克廔膜衣錠):起始用法為每日400-600mg,穩定後可降低至每日200-400mg,分兩次隨餐使用。須特別小心視力及色覺的變化,並配合醫師定期做眼睛的檢查。這兩種藥品優點為較免疫抑制劑安全,可用於中度的類風溼性關節炎治療。 |
|
Rheumatoid Arthritis Educational Session (USA)
|
Rheumatoid Arthritis: Part Two (2008)
|
|
Assoc. Prof Stephen Hall on Rheumatoid Arthritis (UK)
|
|
Rheumatoid Arthritis (類風濕關節炎)
Rheumatoid arthritis (RA) is an autoimmune disease that results in a chronic, systemic inflammatory disorder that may affect many tissues and organs, but principally attacks flexible (synovial) joints. It can be a disabling and painful condition, which can lead to substantial loss of functioning and mobility if not adequately treated. The process involves an inflammatory response of the capsule around the joints (synovium) secondary to swelling (turgescence) of synovial cells, excess synovial fluid, and the development of fibrous tissue (pannus) in the synovium. The pathology of the disease process often leads to the destruction of articular cartilage and ankylosis (fusion) of the joints. RA can also produce diffuse inflammation in the lungs, the membrane around the heart (pericardium), the membranes of the lung (pleura), and white of the eye (sclera), and also nodular lesions, most common in subcutaneous tissue. Although the cause of RA is unknown, autoimmunity plays a big part, and RA is a systemic autoimmune disease. It is a clinical diagnosis made on the basis of symptoms, physical exam, radiographs (X-rays) and labs. Treatments are pharmacological and non-pharmacological. Non-pharmacological treatment includes physical therapy, orthoses, occupational therapy and nutritional therapy but these don't stop the progression of joint destruction. Analgesia (painkillers) and anti-inflammatory drugs, including steroids, suppress symptoms, but don't stop the progression of joint destruction either. Disease-modifying antirheumatic drugs (DMARDs) slow or halt the progress of the disease. The newer biologics are DMARDs. The evidence for complementary and alternative medicine (CAM) treatments for RA related pain is weak, with the lack of high quality evidence leading to the conclusions that their use is currently not supported by the evidence nor proved to be of benefit. About 0.6% of the United States adult population has RA, women two to three times as often as men. Onset is most frequent during middle age, but people of any age can be affected. The name is based on the term "rheumatic fever", an illness which includes joint pain and is derived from the Greek word rheuma (nom.), rheumatos (gen.) ("flow, current"). The suffix -oid ("resembling") gives the translation as joint inflammation that resembles rheumatic fever. The first recognized description of RA was made in 1800 by Dr. Augustin Jacob Landré-Beauvais (1772–1840) of Paris. |
|
Causes (造成因素)
RA is a form of autoimmunity, the causes of which are still not completely known. It is a systemic (whole body) disorder principally affecting synovial tissues. There is no evidence that physical and emotional effects or stress could be a trigger for the disease. The many negative findings suggest that either the trigger varies, or that it might in fact be a chance event inherent with the immune response Half of the risk for RA is believed to be genetic. It is strongly associated with the inherited tissue type major histocompatibility complex (MHC) antigen HLA-DR4 (most specifically DR0401 and 0404), and the genes PTPN22 and PADI4 - -hence family history is an important risk factor. Inheriting the PTPN22 gene has been shown to double a person's susceptibility to RA. PADI4 has been identified as a major risk factor in people of Asian descent, but not in those of European descent. First-degree relatives prevalence rate is 2–3% and disease genetic concordance in monozygotic twins is approximately 15–20%. Smoking is the most significant non-genetic risk with RA being up to three times more common in smokers than non-smokers, particularly in men, heavy smokers, and those who are rheumatoid factor positive. Modest alcohol consumption may be protective. Epidemiological studies have confirmed a potential association between RA and two herpesvirus infections:Epstein-Barr virus (EBV) and Human Herpes Virus 6 (HHV-6). Individuals with RA are more likely to exhibit an abnormal immune response to EBV and have high levels of anti-EBV antibodies. Vitamin D deficiency is common in those with RA and may be causally associated. Some trials have found a decreased risk for RA with vitamin D supplementation while others have not. Pathophysiology The key pieces of evidence relating to pathogenesis are: 1. A genetic link with HLA-DR4 and related allotypes of MHC Class II and the T cell-associated protein PTPN22. 2. An undeniable link to the pathogenesis of vascular disease of many types, including the possibility of a strong causal connection to rheumatoid vasculitis, a typical feature of this condition. 3. A remarkable deceleration of disease progression in many cases by blockade of the cytokine TNF (alpha). 4. A similar dramatic response in many cases to depletion of B lymphocytes, but no comparable response to depletion of T lymphocytes. 5. A more or less random pattern of whether and when predisposed individuals are affected. 6. The presence of autoantibodies to IgGFc, known as rheumatoid factors (RF), and antibodies to citrullinated peptides (ACPA). These data suggest that the disease involves abnormal B cell–T cell interaction, with presentation of antigens by B cells to T cells via HLA-DR eliciting T cell help and consequent production of RF and ACPA. Inflammation is then driven either by B cell or T cell products stimulating release of TNF and other cytokines. The process may be facilitated by an effect of smoking on citrullination but the stochastic (random) epidemiology suggests that the rate limiting step in genesis of disease in predisposed individuals may be an inherent stochastic process within the immune response such as immunoglobulin or T cell receptor gene recombination and mutation. (See entry under autoimmunity for general mechanisms.) If TNF release is stimulated by B cell products in the form of RF or ACPA-containing immune complexes, through activation of immunoglobulin Fc receptors, then RA can be seen as a form of Type III hypersensitivity. If TNF release is stimulated by T cell products such as interleukin-17 it might be considered closer to type IV hypersensitivity although this terminology may be getting somewhat dated and unhelpful. The debate on the relative roles of immune complexes and T cell products in inflammation in RA has continued for 30 years. There is little doubt that both B and T cells are essential to the disease. However, there is good evidence for neither cell being necessary at the site of inflammation. This tends to favour immune complexes (based on antibody synthesised elsewhere) as the initiators, even if not the sole perpetuators of inflammation. Moreover, work by Thurlings and others in Paul-Peter Tak's group and also by Arthur Kavanagh's group suggest that if any immune cells are relevant locally they are the plasma cells, which derive from B cells and produce in bulk the antibodies selected at the B cell stage. Although TNF appears to be the dominant, other cytokines (chemical mediators) are likely to be involved in inflammation in RA. Blockade of TNF does not benefit all patients or all tissues (lung disease and nodules may get worse). Blockade of IL-1, IL-15 and IL-6 also have beneficial effects and IL-17 may be important. Constitutional symptoms such as fever, malaise, loss of appetite and weight loss are also caused by cytokines released into the blood stream. As with most autoimmune diseases, it is important to distinguish between the cause(s) that trigger the process, and those that may permit it to persist and progress. Abnormal Immune Response The factors that allow an abnormal immune response, once initiated, to become permanent and chronic, are becoming more clearly understood. The genetic association with HLA-DR4, as well as the newly discovered associations with the gene PTPN22 and with two additional genes, all implicate altered thresholds in regulation of the adaptive immune response. It has also become clear from recent studies that these genetic factors may interact with the most clearly defined environmental risk factor for RA, namely cigarette smoking. Other environmental factors also appear to modulate the risk of acquiring RA, and hormonal factors in the individual may explain some features of the disease, such as the higher occurrence in women, the not-infrequent onset after child-birth, and the (slight) modulation of disease risk by hormonal medications. Exactly how altered regulatory thresholds allow the triggering of a specific autoimmune response remains uncertain. However, one possibility is that negative feedback mechanisms that normally maintain tolerance of self are overtaken by aberrant positive feedback mechanisms for certain antigens such as IgG Fc (bound by RF) and citrullinated fibrinogen (bound by ACPA). Once the abnormal immune response has become established (which may take several years before any symptoms occur), plasma cells derived from B lymphocytes produce rheumatoid factors and ACPA of the IgG and IgM classes in large quantities. These are not deposited in the way that they are in systemic lupus. Rather, they activate macrophages through Fc receptor and complement binding, which seems to play an important role in the intense inflammatory response present in RA. This contributes to inflammation of the synovium, in terms of edema, vasodilation and infiltration by activated T-cells (mainly CD4 in nodular aggregates and CD8 in diffuse infiltrates). Synovial macrophages and dendritic cells further function as antigen presenting cells by expressing MHC class II molecules, leading to an established local immune reaction in the tissue. The disease progresses in concert with formation of granulation tissue at the edges of the synovial lining (pannus) with extensive angiogenesis and production of enzymes that cause tissue damage. Modern pharmacological treatments of RA target these mediators. Once the inflammatory reaction is established, the synovium thickens, the cartilage and the underlying bone begins to disintegrate and evidence of joint destruction accrues. |
|
Diagnosis
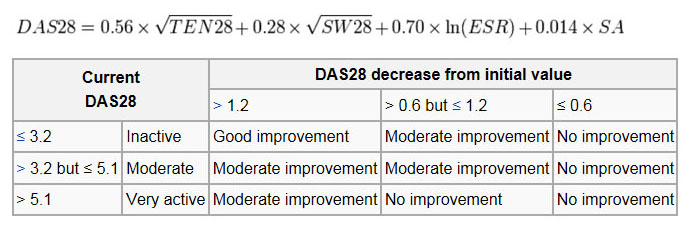
(1) Imaging Signs of destruction and inflammation on ultrasonography and magnetic resonance imaging in the second metacarpophalangeal joint in established RA. Thin arrows indicate an erosive change; thick arrows indicate synovitis. Ultrasonography (left side of image) in the (a) longitudinal and (b) the transverse planes shows both signs of destruction and inflammation. Axial T1-weighted magnetic resonance images were obtained (c) before and (d) after contrast administration, also demonstrating synovitis. Additionally, a coronal T1-weighted magnetic resonance image (e) before contrast administration visualizes the same bone erosion as shown in panels c and d. X-rays of the hands and feet are generally performed in people with a polyarthritis. In RA, there may be no changes in the early stages of the disease, or the x-ray may demonstrate juxta-articular osteopenia, soft tissue swelling and loss of joint space. As the disease advances, there may be bony erosions and subluxation. X-rays of other joints may be taken if symptoms of pain or swelling occur in those joints. Other medical imaging techniques such as magnetic resonance imaging (MRI) and ultrasound are also used in RA. There have been technical advances in ultrasonography. High-frequency transducers (10 MHz or higher) have improved the spatial resolution of ultrasound images; these images can depict 20% more erosions than conventional radiography. Also, color Doppler and power Doppler ultrasound, which show vascular signals of active synovitis depending on the degree of inflammation, are useful in assessing synovial inflammation. This is important, since in the early stages of RA, the synovium is primarily affected, and synovitis seems to be the best predictive marker of future joint damage . (2) Blood Tests When RA is clinically suspected, immunological studies are required, such as testing for the presence of rheumatoid factor (RF, a non-specific antibody). A negative RF does not rule out RA; rather, the arthritis is called seronegative. This is the case in about 15% of patients. During the first year of illness, rheumatoid factor is more likely to be negative with some individuals converting to seropositive status over time. RF is also seen in other illnesses, for example Sjögren's syndrome, Hepatitis C, chronic infections and in approximately 10% of the healthy population, therefore the test is not very specific. Because of this low specificity, new serological tests have been developed, which test for the presence of the anti-citrullinated protein antibodies (ACPAs) or anti-CCP. Like RF, these tests are positive in only a proportion (67%) of all RA cases, but are rarely positive if RA is not present, giving it a specificity of around 95%. As with RF, there is evidence for ACPAs being present in many cases even before onset of clinical disease. The most common tests for ACPAs are the anti-CCP (cyclic citrullinated peptide) test and the Anti-MCV assay (antibodies against mutated citrullinated Vimentin). Recently a serological point-of-care test (POCT) for the early detection of RA has been developed. This assay combines the detection of rheumatoid factor and anti-MCV for diagnosis of RA and shows a sensitivity of 72% and specificity of 99.7%. Also, several other blood tests are usually done to allow for other causes of arthritis, such as lupus erythematosus. The erythrocyte sedimentation rate (ESR), C-reactive protein, full blood count, renal function, liver enzymes and other immunological tests (eg , antinuclear antibody / ANA) are all performed at this stage. Elevated ferritin levels can reveal hemochromatosis, a mimic of RA, or be a sign of Still's disease, a seronegative, usually juvenile, variant of rheumatoid arthritis. (3) Criteria In 2010 the 2010 ACR / EULAR Rheumatoid Arthritis Classification Criteria were introduced. These new classification criteria overruled the "old" ACR criteria of 1987 and are adapted for early RA diagnosis. The "new" classification criteria, jointly published by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) establish a point value between 0 and 10. Every patient with a point total of 6 or higher is unequivocally classified as an RA patient, provided he has synovitis in at least one joint and given that there is no other diagnosis better explaining the synovitis. Four areas are covered in the diagnosis: ★ joint involvement, designating the metacarpophalangeal joints, proximal interphalangeal joints, the interphalangeal joint of the thumb, second through fifth metatarsophalangeal joint and wrist as small joints, and shoulders, elbows, hip joints, knees, and ankles as large joints: ● Involvement of 1 large joint gives 0 points ● Involvement of 2-10 large joints gives 1 point ● Involvement of 1-3 small joints (with or without involvement of large joints) gives 2 points ● Involvement of 4-10 small joints (with or without involvement of large joints) gives 3 points ● Involvement of more than 10 joints (with involvement of at least 1 small joint) gives 5 points ★ serological parameters - including the rheumatoid factor as well as ACPA - "ACPA" stands for "anti-citrullinated protein antibody": ● Negative RF and negative ACPA gives 0points ● Low-positive RF or low-positive ACPA gives 2 points ● High-positive RF or high-positive ACPA gives 3 points ★ acute phase reactants: 1 point for elevated erythrocyte sedimentation rate, ESR, or elevated CRP value (c-reactive protein) ★ duration of arthritis: 1 point for symptoms lasting six weeks or longer The new criteria accommodate to the growing understanding of RA and the improvements in diagnosing RA and disease treatment. In the "new" criteria serology and autoimmune diagnostics carries major weight, as ACPA detection is appropriate to diagnose the disease in an early state, before joints destructions occur. Destruction of the joints viewed in radiological images was a significant point of the ACR criteria from 1987. This criterion no longer is regarded to be relevant, as this is just the type of damage that treatment is meant to avoid. The criteria are not intended for the diagnosis for routine clinical care; they were primarily intended to categorize research (classification criteria). In clinical practice, the following criteria apply: ● two or more swollen joints ● morning stiffness lasting more than one hour for at least six weeks ● the detection of rheumatoid factors or autoantibodies against ACPA such as autoantibodies to mutated citrullinated vimentin can confirm the suspicion of RA. A negative autoantibody result does not exclude a diagnosis of RA. Differential diagnoses Several other medical conditions can resemble RA, and usually need to be distinguished from it at the time of diagnosis: ● Crystal induced arthritis (gout, and pseudogout) - usually involves particular joints (knee, MTP1, heels) and can be distinguished with aspiration of joint fluid if in doubt. Redness, asymmetric distribution of affected joints, pain occurs at night and the starting pain is less than an hour withgout. ● Osteoarthritis - distinguished with X-rays of the affected joints and blood tests, age (mostly older patients), starting pain less than an hour, a-symmetric distribution of affected joints and pain worsens when using joint for longer periods. ● Systemic lupus erythematosus (SLE) - distinguished by specific clinical symptoms and blood tests (antibodies against double-stranded DNA) ● One of the several types of psoriatic arthritis resembles RA - nail changes and skin symptoms distinguish between them ● Lyme disease causes erosive arthritis and may closely resemble RA - it may be distinguished by blood test in endemic areas ● Reactive arthritis (previously Reiter's disease) - asymmetrically involves heel, sacroiliac joints, and large joints of the leg. It is usually associated with urethritis, conjunctivitis, iritis, painless buccal ulcers, and keratoderma blennorrhagica. ● Ankylosing spondylitis - this involves the spine, although a RA-like symmetrical small-joint polyarthritis may occur in the context of this condition. ● Hepatitis C - RA-like symmetrical small-joint polyarthritis may occur in the context of this condition. Hepatitis C may also induce Rheumatoid Factor auto-antibodies Rarer causes that usually behave differently but may cause joint pains: ● Sarcoidosis, amyloidosis, and Whipple's disease can also resemble RA. ● Hemochromatosis may cause hand joint arthritis. ● Acute rheumatic fever can be differentiated from RA by a migratory pattern of joint involvement and evidence of antecedent streptococcal infection. Bacterial arthritis (such as streptococcus) is usually asymmetric, while RA usually involves both sides of the body symmetrically. ● Gonococcal arthritis (another bacterial arthritis) is also initially migratory and can involve tendons around the wrists and ankles. Monitoring Progression The progression of RA can be followed using scores such as Disease Activity Score of 28 joints (DAS28). It is widely used as an indicator of RA disease activity and response to treatment, but is not always a reliable indicator of treatment effect. The joints included in DAS28 are (bilaterally): proximal interphalangeal joints (10 joints), metacarpophalangeal joints (10), wrists (2), elbows (2), shoulders (2) and knees (2). When looking at these joints , both the number of joints with tenderness upon touching (TEN28) and swelling (SW28) are counted. In addition, the erythrocyte sedimentation rate (ESR) is measured. Also, the patient makes a subjective assessment (SA) of disease activity during the preceding 7 days on a scale between 0 and 100, where 0 is "no activity" and 100 is "highest activity possible". With these parameters, DAS28 is calculated as: |
|
Management
There is no cure for RA, but treatments can improve symptoms and slow the progress of the disease. Disease-modifying treatment has the best results when it is started early and aggressively. The goals of treatment are to minimize symptoms such as pain and swelling, to prevent bone deformity (for example, bone erosions visible in X-rays), and to maintain day-to-day functioning. This can often be achieved using two main classes of medications: analgesics such as NSAIDs, and disease-modifying antirheumatic drugs (DMARDs). RA should generally be treated with at least one specific anti-rheumatic medication. The use of benzodiazepines (such as diazepam) to treat the pain is not recommended as it does not appear to help and is associated with risks. Analgesics, other than NSAIDs, offer lesser, but some benefit with respect to pain. (1) Lifestyle Regular exercise is recommended as both safe and useful to maintain muscles strength and overall physical function. It is uncertain if specific dietary measures have an effect. (2) Disease Modifying Agents Disease-modifying antirheumatic drugs (DMARD) are the primary treatment for RA. They are a diverse collection of drugs, grouped by use and convention. They have been found to improve symptoms, decrease joint damage, and improve overall functional abilities. They should be started very early in the disease as when they result in disease remission in approximately half of people and improved outcomes overall. The most commonly used agent is methotrexate with other frequently used agents including sulfasalazine and leflunomide. Sodium aurothiomalate (Gold) and cyclosporin are less commonly used due to more common adverse effects. Agents may be used in combinations. Methotrexate is the most important and useful DMARD and is usually the first treatment. Adverse effects should be monitored regularly with toxicity including gastrointestinal, hematologic, pulmonary, and hepatic. The most common undesirable affect is that it increases liver enzymes in almost 15% of people. It is thus recommended that those who consistently demonstrate abnormal levels of liver enzymes or have a history of liver disease or alcohol use undergo liver biopsies. Methotrexate is also considered a teratogenic and as such, it is recommended women of childbearing age should use contraceptives to avoid pregnancy and to discontinue its use if pregnancy is planned. Biological agents should generally only be used if methotrexate and other conventional agents are not effective after a trial of three months. These agents include: tumor necrosis factor alpha (TNFα) blockers such as infliximab; interleukin 1 blockers such as anakinra, monoclonal antibodies against B cells such as rituximab, T cell costimulation blocker such as abatacept among others. They are often used in combination with either methotrexate or leflunomide. TNF blockers and methotrexate appear to have similar effectiveness when used alone and better results are obtained when used together. TNF blockers appear to have equivalent effectiveness with etanercept appearing to be the safest. Abatacept appears effective for RA with 20% more people improving with treatment than without. There however is a lack of evidence to distinguish between the biologics available for RA. Issues with the biologics include their high cost and association with infections including tuberculosis. (3) Anti-inflammatory agents NSAIDs reduce both pain and stiffness in those with RA. Generally they appear to have no effect on people's long term disease course and thus are no longer first line agents. NSAIDs should be used with caution in those with gastrointestinal, cardiovascular, or kidney problems. COX-2 inhibitors, such as celecoxib, and NSAIDs are equally effective. They have a similar gastrointestinal risk as an NSAIDs plus a proton pump inhibitor. In the elderly there is less gastrointestinal intolerance to celecoxib than to NSAIDs alone. There however is an increased risk of myocardial infarction with COX-2 inhibitors. Anti-ulcer medications are not recommended routinely but only in those high risk of gastrointestinal problems. Glucocorticoids can be used in the short term for flare-ups, while waiting for slow-onset drugs to take effect. Injection of glucocorticoids into individual joints is also effective. While long-term use reduces joint damage it also results in osteoporosis and susceptibility to infections, and thus is not recommended. (4) Surgery In early phases of the disease, an arthroscopic or open synovectomy may be performed. It consists of the removal of the inflamed synovia and prevents a quick destruction of the affected joints. Severely affected joints may require joint replacement surgery, such as knee replacement. Postoperatively, physiotherapy is always necessary. (5) Alternative Medicine There has been an increasing interest in the use of complementary and alternative medicine interventions for the treatment of pain in rheumatoid arthritis. While there have been multiple studies showing beneficial effects in RA on a wide variety of CAM modalities, these studies are often affected by publication bias and are generally not high quality evidence such as randomized controlled trials (RCTs), making definitive conclusions difficult to reach. The National Center for Complementary and Alternative Medicine has concluded, "In general, there is not enough scientific evidence to prove that any complementary health approaches are beneficial for RA, and there are safety concerns about some of them. Some mind and body practices and dietary supplements may help people with RA manage their symptoms and therefore may be beneficial additions to conventional RA treatments, but there is not enough evidence to draw conclusions." A systematic review of CAM modalities (excluding fish oil) found "The major limitation in reviewing the evidence for CAMs is the paucity of RCTs in the area. The available evidence does not support their current use in the management of RA." One review suggests that of the various alternative medicine treatments evaluated, only acupuncture, bee venom acupuncture, herbal remedies, dietary omega-3 fatty acids, and pulsed electromagnetic field therapy have been studied with RCTs and show promise in treating the pain of RA, though no definitive conclusions could be reached. (6) Dietary Supplements The American College of Rheumatology states that no herbal medicines have health claims supported by high quality evidence and thus they do not recommend their use. There is no scientific basis to suggest that herbal supplements advertised as "natural" are safer for use than conventional medications as both are chemicals. Herbal medications, although labelled "natural", may be toxic or fatal if consumed. Some evidence supports omega-3 fatty acids and gamma-linolenic acid in RA. The benefit from omega-3 appears modest but consistent, though the current evidence is not strong enough to determine that supplementation with omega-3 polyunsaturated fatty acids (found in fish oil) is an effective treatment for RA. Gamma-linolenic acid, which may reduce pain, tender joint count and stiffness, is generally safe. The following show promise as treatments for RA, based on preliminary studies: boswellic acid, curcumin, Devil's claw, Euonymus alatus, and Thunder god vine (Tripterygium wilfordii). Herbal supplements can often have significant side effects, and can interact with prescription medications being taken at the same time. These risks are often exaggerated by the false general belief by patients that herbal supplements are always safe and the hesitancy by patients in reporting the use of herbal supplements to physicians. NCCAM has noted that, "In particular, the herb thunder god vine (Tripterygium wilfordii) can have serious side effects." |