現在位置 : 病症 > 癌症 > 癌症免疫療法 - Cancer Immunotherapy
|
Click here to edit.
|
|
癌症免疫療法,評為年度顯學
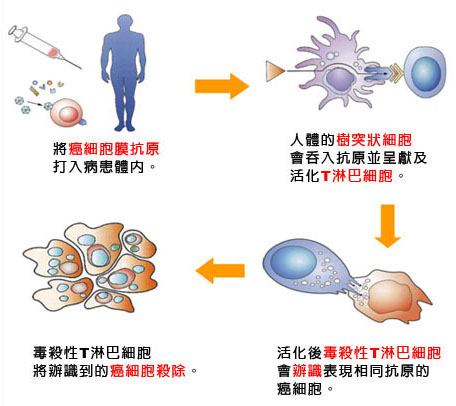
台灣醒報 ╱記者李昀澔 台北報導 2013.12.23 11:08 am 癌症免疫療法是目前醫學研究的「顯學」!現階段的癌症治療,都是透過「外力」消滅腫瘤細胞、防止擴散及轉移為主,免疫療法則是另闢蹊徑,促進病人免疫系統功能,也就是讓病人「自體」產生消滅癌細胞的能力;由於癌症免疫療法極具發展潛力,因而被全球最頂尖的學術期刊《科學》雜誌評選為年度「學術重大突破」。 全球研究顯學 《科學》雜誌編輯部將癌症免疫療法列為未來臨床治療趨勢的主因,是由於各種癌症致病原因各不相同,但全球已有許多團隊投入各種癌症免疫療法的研究,都獲得正面的實驗結果。 《科學》雜誌新聞部總編輯提姆阿本塞勒表示,儘管距離臨床普遍應用還有很長的距離,但細胞與動物實驗,以及部分小規模的人體實驗結果,都顯示此類對抗癌細胞的新思維,確實能夠有效消滅癌細胞。 改變白血球免疫功能 其中一種熱門的癌症免疫療法,是促進一種稱為T細胞的白血球功能;主要策略是透過改變T細胞表面帶有的基因,讓T細胞能夠自動辨認某種類型的癌細胞,進而啟動攻擊機制,達到殺死癌細胞的目地。 今年3月,美國紐約史隆凱特琳紀念癌症中心發表於《科學:轉譯醫學》的研究發現,在5名「B細胞急性淋巴母細胞白血病」患者,接受T細胞免疫療法後,其中3人的腫瘤體積明顯縮小。 另一組美國賓州大學與史隆凱特琳紀念癌症中心合作的研究團隊,日前則發表一組75人的臨床人體實驗結果,其中45人在T細胞功能啟動後,腫瘤明顯縮小,但部分患者後續仍有腫瘤復發情形。 控制白血球表面抗原 另一種癌症免疫療法,則是控制部分T細胞表面的抗原,例如CTLA-4或PD-1等,主要功能在於「抑制」T細胞持續進行免疫反應,以避免傷害正常細胞的抗原,也就是利用抗體阻擋這類抗原的作用之後,T細胞的免疫反應就不會被「關閉」,能夠持續進行消滅癌細胞的任務。 耶魯大學團隊在今年發表於《科學》的研究指出,使用PD-1抗原的抗體治療300名各種癌症患者後,發現有17%的肺癌、29%的胰臟癌、31%的黑色素癌,也就是皮膚癌患者,其體內腫瘤體積至少縮小50%。 「癌症免疫療法目前遇到的困境是,無法解釋為何不是所有接受免疫療法的同種類病人都有效?」《科學》雜誌撰稿人珍妮佛庫辛法蘭柯兒解釋,癌症免疫療法並不像現行的標靶治療,必須符合特定的基因型才能產生藥效,而是增強或開啟病人本身免疫系統的特定功能。 就理論上來說,相同種類的腫瘤細胞都會有一定程度的減少,但人體實驗結果卻對部分病人完全無效,因此仍有待進一步研究釐清。 |
|
Cancer Immunotherapy
Cancer immunotherapy is the use of the immune system to reject cancer. The main premise is stimulating the patient's immune system to attack the malignant tumor cells that are responsible for the disease. This can be either through immunization of the patient (e.g., by administering a cancer vaccine, such as Dendreon's Provenge), in which case the patient's own immune system is trained to recognize tumor cells as targets to be destroyed, or through the administration of therapeutic antibodies as drugs, in which case the patient's immune system is recruited to destroy tumor cells by the therapeutic antibodies. Cell based immunotherapy is another major entity of cancer immunotherapy. This involves immune cells such as the Natural killer Cells (NK cells), Lymphokine Activated killer cell(LAK), Cytotoxic T Lymphocytes(CTLs), Dendritic Cells (DC), etc., which are either activated in vivo by administering certain cytokines such as Interleukins or they are isolated, enriched and transfused to the patient to fight against cancer. Since the immune system responds to the environmental factors it encounters on the basis of discrimination between self and non-self, many kinds of tumor cells that arise as a result of the onset of cancer are more or less tolerated by the patient's own immune system since the tumor cells are essentially the patient's own cells that are growing, dividing and spreading without proper regulatory control. In spite of this fact, however, many kinds of tumor cells display unusual antigens that are either inappropriate for the cell type and/or its environment, or are only normally present during the organisms' development (e.g. fetal antigens). Examples of such antigens include the glycosphingolipid GD2, a disialoganglioside that is normally only expressed at a significant level on the outer surface membranes of neuronal cells, where its exposure to the immune system is limited by the blood–brain barrier. GD2 is expressed on the surfaces of a wide range of tumor cells including neuroblastoma, medulloblastomas, astrocytomas, melanomas, small-cell lung cancer, osteosarcomas and other soft tissue sarcomas. GD2 is thus a convenient tumor-specific target for immunotherapies. Other kinds of tumor cells display cell surface receptors that are rare or absent on the surfaces of healthy cells, and which are responsible for activating cellular signal transduction pathways that cause the unregulated growth and division of the tumor cell. Examples include ErbB2, a constitutively active cell surface receptor that is produced at abnormally high levels on the surface of breast cancer tumor cells. The use of some agents can lead to the re-activation of latent tuberculosis (TB) and this must be assessed for before those agents are used therapeutically. History Cancer immunotherapy has arisen from advances in both oncology and immunology fields over the last few centuries. Immunotherapy began in 1796 when Edward Jenner produced the first vaccine involving immunisation with cowpox to prevent smallpox. Towards the end of the 19th century Emil von Behring and Shibasabo Kitasato discovered that injecting animals with diphtheria toxin produced blood serum with anti-toxins to it. Following this Paul Ehrlich's research gave rise to the "magic bullet" concept; using antibodies to specifically target a disease. The production of pure monoclonal antibodies for therapeutic use was not available until 1975 when Georges J. F. Köhler and Cesar Milstein produced the hybridoma technology, although it wasn't until 1997 when Rituximab, the first antibody treatment for cancer, was approved by the FDA for treatment of follicular lymphoma. Since this approval, 11 other antibodies have been approved for cancer; Trastuzumab (1998), Gemtuzumab ozogamicin (2000), Alemtuzumab (2001), Ibritumomab tiuxetan (2002), Tositumomab (2003), Cetuximab (2004), Bevacizumab (2004), Panitumumab (2006), Ofatumumab (2009), Ipilimumab (2011) and Brentuximab vedotin (2011). The production of vaccines for cancer came later than the use of monoclonal antibodies. As our understanding of human immunology has improved, so has our potential to produce effective cancer vaccines. The first cell-based immunotherapy cancer vaccine, Sipuleucel-T, was approved in 2010 for the treatment of prostate cancer. |
|
Cell-Based immunotherapy
Adoptive T-cell therapy Cancer specific T-cells can be obtained by fragmentation and isolation of tumour infiltrating lymphocytes, or by genetically engineering cells from peripheral blood. The cells are activated and grown prior to transfusion into the recipient (tumour bearer). Adoptive T-cell therapy is form of passive immunization by the transfusion of T-cells, which are cells of the immune system. They are found in blood and tissue and usually activate when they find foreign pathogens. Specifically they activate when the T-cell's surface receptors encounter other cells that display small parts of foreign proteins on their surface MHC molecules, known as antigens. These can be either infected cells, or specialised immune cells known as antigen presenting cells (APCs). They are found in normal tissue and in tumor tissue, where they are known as tumor infiltrating lymphocytes (TILs). They are activated by the presence of APCs, such as dendritic cells that present tumor antigens to the T-cells. Although these cells have the capability of attacking the tumor, the environment within the tumor is highly immunosuppressive, preventing immune-mediated tumour death. There are multiple ways of producing and obtaining tumour targeted T-cells. T-cells specific to a tumor antigen can either be removed from a tumor sample (TILs) or T-cells can be removed from the blood and genetically engineered to be tumor specific. Subsequent activation and expansion of these cells is performed outside the body (ex vivo) and then they are transfused into the recipient. Although research has made major advances in this form of therapy, there is no approved adoptive T-cell therapy as yet. The tumor specific T-cells used for treatment will be specific for a particular antigen present within the tumor, or for the stroma or vasculature, which the tumor may be dependent on. Examples of T-cell targets are tissue differentiation antigens, mutant protein antigens, oncogenic viral antigens, cancer-testis antigens and vascular or stromal specific antigens. Tissue differentiation antigens are those that are specific to a certain type of tissue. T-cells specific to these antigens will target normal cells that contain these antigens as well as cancer cells (e.g. carcinoembryonic antigen; CEA). Mutant protein antigens are likely to be much more specific to cancer cells because normal cells shouldn't contain these proteins. Normal cells will display the normal protein antigen on their MHC molecules, whereas cancer cells will display the mutant version. T-cells can differentiate between these two, selectively targeting the cancer cell. Some viral proteins are implicated in forming cancer (oncogenesis), and therefore T-cells that are specific to viral antigens can be used to attack infected cells (which will include cancer cells). Cancer-testis antigens are antigens expressed primarily in the germ cells of the testes, but also in fetal ovaries and the trophoblast. Some cancer cells aberrantly express these proteins and therefore present these antigens, allowing attack by T-cells specific to these antigens. Example antigens of this type are CTAG1B and MAGEA1. Blood cells are removed from the body, incubated with tumour antigen(s) and activated. Mature dendritic cells are then returned to the original cancer-bearing donor to induce an immune response. Dendritic cell therapy Dendritic cell therapy comprises a group of methods that provoke anti-tumor responses by causing dendritic cells to present tumor antigens. Dendritic cells present antigens to lymphocytes, which activates them, priming them to kill cells which also present the antigen. They are utilised in cancer treatment to specifically target cancer antigens. This group of cell-based therapy boasts the only approved treatment for cancer, Sipuleucel-T. One method of inducing dendritic cells to present tumor antigens is by vaccination with short peptides (small parts of protein that correspond to the protein antigens on cancer cells). These peptides on their own do not stimulate a strong immune response and may be given in combination with highly immunogenic substances known as adjuvants. This provokes a strong response to the adjuvant being used, while also producing a (sometimes) robust anti-tumor response by the immune system. Other adjuvants being used are proteins or other chemicals that attract and/or activate dendritic cells, such as granulocyte macrophage colony-stimulating factor (GM-CSF). Dendritic cells can also be activated within the body (in vivo) by making tumour cells to express (GM-CSF). This can be achieved by either genetically engineering tumor cells that produce GM-CSF or by infecting tumor cells with an oncolytic virus that expresses GM-CSF. Another strategy used in dendritic cell therapy is to remove dendritic cells from the blood of a person with cancer and activate them outside the body (ex vivo). The dendritic cells are activated in the presence of tumor antigens, which may be a single tumor specific peptide/protein or a tumor cell lysate (a solution of broken down tumor cells). These activated dendritic cells are put back into the body where they provoke an immune response to the cancer cells. Adjuvants are sometimes used systemically to increase the anti-tumor response provided by ex vivo activated dendritic cells. More modern dendritic cell therapies include the use of antibodies that bind to receptors on the surface of dendritic cells. Antigens can be added to the antibody and can induce the dendritic cells to mature and provide immunity to the tumor. Dendritic cell receptors such as TLR3, TLR7, TLR8 or CD40 have been used as targets by antibodies to produce immune responses. Sipuleucel-T Sipuleucel-T (Provenge) is the first approved cancer vaccine. It was approved for treatment of asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer in 2010. The treatment consists of removal of antigen presenting cells from blood by leukapheresis, and growing them with the fusion protein P2024 made from GM-CSF and prostatic acid phosphatase (PAP). These cells are infused back into the recipient to induce an immune response against the tumor because the PAP protein is prostate specific. This process is repeated three times. Monoclonal antibody therapy Antibodies are a key component of the adaptive immune response, playing a central role in both in the recognition of foreign antigens and the stimulation of an immune response to them. It is not surprising therefore, that many immunotherapeutic approaches involve the use of antibodies. The advent of monoclonal antibody technology has made it possible to raise antibodies against specific antigens such as the unusual antigens that are presented on the surfaces of tumors. Types of monoclonal antibodies Two types of monoclonal antibodies are used in cancer treatments: Naked monoclonal antibodies are antibodies without modification. Most of the currently used antibodies therapies fall into this category. Conjugated monoclonal antibodies are joined to another molecule, which is either toxic to cells or radioactive. The toxic chemicals are usually routinely used chemotherapy drugs but other toxins can be used. The antibody binds to specific antigens on the surface of cancer cells and directs the drug or radiation to the tumor. Radioactive compound-linked antibodies are referred to as radiolabelled. If the antibodies are labelled with chemotherapy or toxins, they are known as chemolabelled or immunotoxins, respectively. Antibodies are also referred to as murine, chimeric, humanized and human. Murine antibodies were the first type of antibody to be produced, and they carry a great risk of immune reaction by the recipient because the antibodies are from a different species. Chimeric antibodies were the first attempt to reduce the immunogenicity of these antibodies. They are murine antibodies with a specific part of the antibody replaced with the corresponding human counterpart, known as the constant region. Humanized antibodies are almost completely human; only the complementarity determining regions of the variable regions are derived from murine antibodies. Human antibodies have a completely human amino acid sequence. |