現在位置 : RA > 生物製劑 - 復邁 (Humira, Adalimumab)
|
Adalimumab (Humira)
Adalimumab (Humira) is a disease-modifying antirheumatic drug (DMARD) labeled for the adult treatment of moderately to severely active rheumatoid arthritis. It is a recombinant human immunoglobulin G1 monoclonal antibody that specifically binds and inhibits the inflammatory cytokine tumor necrosis factor (TNF)α. |
|
生物工程製劑
生物工程製劑包括: ● 腫瘤壞死因子α (TNF-α) 抑制劑:依那西普(Enbrel®)、英利昔單抗(Remicade®)、復邁(Humira®) ● 白質1抑制劑:阿那白滯素 ● 抗CD20單抗:利妥昔單抗(Rituxan®) ● 白質6受體抑制劑:托珠單抗(Actemra®) |
|
Biologic Agents
Biologics have greatly improved the treatment of RA. They work by interrupting certain components of the immune system. One group of biologic drugs works by blocking the inflammatory protein known as tumor necrosis factor. Because these drugs suppress the immune system, infection is among the most serious side effects of these drugs. Anti-TNF biologics are given by injection. Irritation at the site of injection is a common side effect. Anti-TNF drugs impair the immune system, so it’s important to be tested for latent tuberculosis and hepatitis B virus infection before starting treatment. If present, these infections can flare up after treatment begins. The risk of lymphoma and skin cancer may rise with long-term use of these drugs. How Humira Works Rheumatoid arthritis (RA) has been linked to an overproduction of a protein called tumor necrosis factor (TNF). In people with RA, extra TNF causes inflammation in the joints. But HUMIRA — a medication called a TNF blocker — can help block the action of TNF, helping to reduce inflammation and improve symptoms. Because TNF blockers, including HUMIRA, affect the immune system, they can lower the ability to fight infections and may cause other serious side effects. |
|
Biologics: TNF Antagonists for Rheumatoid Arthritis
|
|
Adalimumab (Humira, 復邁)
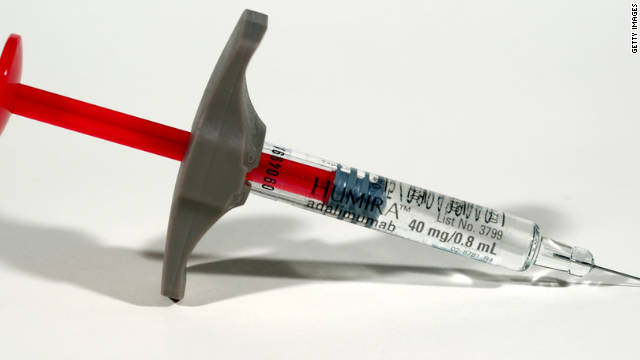
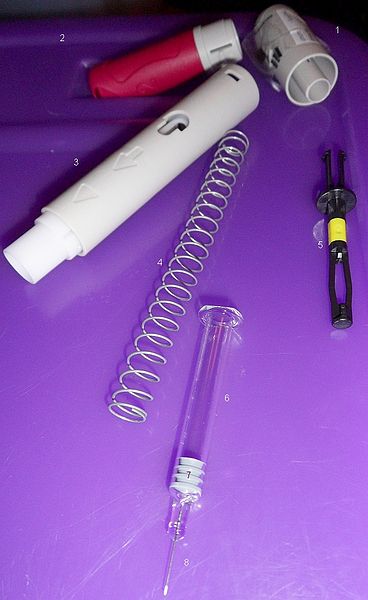
Adalimumab, trade name Humira, is a TNF inhibiting anti-inflammatory drug. AbbVie's trade name is derived from the acronym of "human monoclonal antibody in rheumatoid arthritis" (HUMIRA). Adalimumab binds to tumor necrosis factor-alpha (TNFα), preventing it from activating TNF receptors; TNFα inactivation has proven to be important in downregulating the inflammatory reactions associated with autoimmune diseases. Adalimumab has been approved in the United States for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, moderate to severe chronic psoriasis and juvenile idiopathic arthritis. In rheumatoid arthritis, adalimumab has a response rate similar to methotrexate, and in combination nearly doubles the response rate of methotrexate alone. Because TNFα is part of the immune system that protects the body from infection, treatment with adalimumab may increase the risk of infections. It is marketed in 0.8 mL vials, preloaded 0.8 mL syringes and also in preloaded pen devices (called Humira Pen), injected subcutaneously, typically by the patient at home. It cannot be administered orally because the digestive system would destroy the drug. Adalimumab was the first fully human monoclonal antibody drug approved by the FDA. It was derived from phage display, and was discovered through a collaboration between BASF Bioresearch Corporation (Worcester, Massachusetts, a unit of BASF) and Cambridge Antibody Technology as D2E7, then further manufactured at BASF Bioresearch Corporation and developed by BASF Knoll (BASF Pharma) and, ultimately, manufactured and marketed by Abbott Laboratories after the acquisition of BASF Pharma by Abbott. Humira costs approximately $1,662 per month, like the TNF-alpha inhibitor etanercept (Enbrel). Methotrexate costs approximately $13 to $85 per month. In 2012 Humira drug had $4.3 billion of sales in the US, and $9.3 billion worldwide. |
|
Humira History
Adalimumab was discovered as a result of the collaboration between BASF Bioresearch Corporation (Worcester, Massachusetts, a unit of BASF) and Cambridge Antibody Technology which began in 1993. It was the third TNF inhibitor, after infliximab and etanercept, to be approved in the United States. It was constructed from a fully human monoclonal antibody, while infliximab is a mouse-human chimeric antibody and etanercept is a TNF receptor-IgG fusion protein. The drug candidate was discovered initially using CAT's phage display technology and named D2E7. The key components of the drug were found by guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen TNF alpha. The ultimate clinical candidate, D2E7, was created and manufactured at BASF Bioresearch Corporation and taken through most of the drug development process by BASF Knoll, then further development, manufacturing and marketing by Abbott Laboratories, after Abbott acquired the pharmaceutical arm of BASF Knoll. On 2 January 2013, Abbott Laboratories separated into two independent companies, Abbott and AbbVie. As a result, AbbVie is taking responsibility for the further development and marketing of Humira. As of 2008 adalimumab had been approved by the U.S. Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, moderate to severe chronic psoriasis and juvenile idiopathic arthritis. Although only approved for ulcerative colitis from late 2012 by the FDA in the disease's management, it had been used for several years in cases that have not responded to conventional treatment at standard dosing for Crohn's Disease. Notable clinical and marketing milestones 1999: Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7 2001, June: Results from ARMADA, a double-blind, placebo-controlled clinical trial involving 271 patients with active rheumatoid arthritis despite treatment with methotrexate are announced. Among the results are that 50% of patients show a 50% improvement in ACR score. 2002: Broke ground on a new state-of-the-art biologics manufacturing facility. 2002: Adalimumab results from five separate trials show that it is effective at reducing signs and symptoms of rheumatoid arthritis. In these studies, adalimumab had a rapid onset of action and sustained efficacy. Furthermore, adalimumab was safe and effective when given alone or in combination with MTX as a subcutaneous injection. 2002, December 31: Humira approved by the U.S. Food and Drug Administration (FDA) for treatment of rheumatoid arthritis. 2003: Launched Humira for rheumatoid arthritis and continued clinical studies for additional indications. 2005: Launched Humira for psoriatic arthritis. Exceeded $1 billion in annual sales for the first time. 2005, 10 December: Eisai Submits New Drug Application for Rheumatoid Arthritis Drug Adalimumab (D2E7) in Japan. 2006: Submitted Humira for the Crohn’s disease indication and launched it for AS. Exceeded $2 billion in annual sales. 2007: Launched Humira for Crohn’s disease in the United States, submitted Humira for global regulatory approval for psoriasis — the fifth new Humira disease indication at this time, achieved more than $3 billion in worldwide Humira sales. 2007, 10 December: Abbott Opens New Biotechnology Manufacturing Facility in Puerto Rico[16] 2009, 10 June: Five-Year Data Demonstrate Initial Use of Humira Plus Methotrexate May Prevent Further Joint Damage in Early Rheumatoid Arthritis Patients 2012, 16 March: Humira could be associated with a significant decrease in vascular inflammation, a major risk factor of cardiovascular disease Indication Adalimumab, like other TNF inhibitors, infliximab, etanercept, certolizumab pegol and golimumab, may be used in the treatment of several conditions where the suppression of the immune response is desired. Not all the listed applications have been approved worldwide. Rheumatoid arthritis Adalimumab has been shown to reduce the signs and symptoms of moderate-to-severe rheumatoid arthritis (RA) in adults. It has also been shown to have efficacy in moderate to severe polyarticular juvenile idiopathic arthritis (JIA) in children 4 years of age and older, and is approved for use in the treatment of that condition. In RA it can be used alone or with methotrexate or similar medicines. In the US since 2002 Psoriatic arthritis Adalimumab is undergoing trials for use in treating psoriasis and psoriatic arthritis. Ankylosing spondylitis Adalimumab has been shown to reduce the signs and symptoms of, and is approved for treatment of, ankylosing spondylitis (AS) in adults. Crohn's disease Adalimumab has been shown to reduce the signs and symptoms of, and is approved for treatment of, moderate to severe Crohn's disease. Since 2009 in the UKMorey, Sheralyn. "World Pharmaceutical Market Outlook: UK - Summary of NICE Approvals in September 2009". Retrieved 2014-02-04. Ulcerative colitis Adalimumab may be effective and well tolerated in Ulcerative colitis. Its efficacy in maintaining clinical remission needs to be confirmed in a randomized controlled trial. It has been approved by the FDA for treatment of moderate-to-severe cases in adults. Plaque psoriasis Adalimumab has been shown to treat moderate to severe chronic (lasting a long time) plaque psoriasis (Ps) in adults who have the condition in many areas of their body and who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet light alone or with pills).[26] Adalimumab has been shown to be effective therapy when used either continuously or intermittently in patients with moderate to severe psoriasis. Juvenile idiopathic arthritis Adalimumab has been shown to reduce the signs and symptoms of moderate to severe polyarticular juvenile idiopathic arthritis (JIA) in children four years of age and older. For patients 15 kg (33 lbs) to 30 kg (66 lbs) administer 20 mg subcutaneously every other week. For patients weighing more than 30 kg (66 lbs) administer 40 mg subcutaneously every other week. Safety Because adalimumab suppresses TNF, which is part of the immune system, latent infections, such as tuberculosis, can be reactivated, and the immune system may be unable to fight new infections. This has led to fatal infections. According to the product labeling, after a number of studies and reports of adverse events in patients receiving adalimumab, including serious and sometimes fatal blood disorders, serious infections including tuberculosis (TB) and infections caused by viruses, fungi, or bacteria, rare reports of lymphoma and solid tissue cancers, rare reports of serious liver injury, rare reports of demyelinating central nervous system disorders, rare reports of cardiac failure, the U.S. Food and Drug Administration issued a black box warning to doctors which appears in the product labeling of adalimumab and the other TNF drugs instructing them to screen and monitor potential patients more carefully. Anaphylaxis or serious allergic reactions may occur. |
|
Press Release (by Abbott)
Abbott Announces 10-Year Data Evaluating HUMIRA® (Adalimumab) for Patients with Long-Standing Moderate-to-Severe Rheumatoid Arthritis Studies are Among Longest Open-Label Trials in Patients with Rheumatoid Arthritis Treated with an Anti-TNF Date: June 06, 2012 Berlin — Abbott (NYSE: ABT) today announced results from two long-term, open-label studies, which evaluated the results of HUMIRA® (adalimumab) treatment for up to 10 years in patients with long-standing, moderate-to-severe rheumatoid arthritis (RA). These 10-year HUMIRA studies, DE019 and DE020, are among the longest, open-label trials in RA. Results presented at the European League Against Rheumatism (EULAR) 2012 Congress in Berlin, Germany, include information on clinical response, radiographic inhibition and physical function data in patients with long-standing RA. In both studies, patients were assessed for improvements in signs and symptoms of the disease, such as joint pain, swelling and stiffness, as well as physical function and achievement of clinical remission. Following up to 10 years of HUMIRA therapy, patients in the studies continued to maintain improvements in disease activity. In both DE019 and DE020, a DAS28 (CRP) <2.6, a measure of clinical remission, was observed in more than half of patients who continued on HUMIRA for up to 10 years (59.6 percent and 57.2 percent, respectively). A DAS28 score of <2.6 as a measure of clinical remission is supported by both EULAR and the American College of Rheumatology (ACR). DE019 also assessed the ability of HUMIRA to inhibit radiographic progression. Patients who completed 10 years of treatment and also had X-rays available at baseline and year 10 demonstrated an average change of 2.8 in modified total Sharp score (mTSS), a measure of radiographic inhibition. In this study, patients who were initially treated with HUMIRA plus methotrexate (MTX) for the first year had less radiographic progression (measured as mean change in mTSS) at year 10 compared with patients who initially received placebo plus MTX. This result was driven by the change in mTSS during the one year randomized controlled trial. "Managing RA is more than just treating the signs and symptoms, but also inhibiting joint damage, and improving physical function," said Dr. Edward Keystone, Professor of Medicine, University of Toronto, Canada. "The results of these long-term studies add to the vast arsenal of evidence that rheumatologists can reference when treating their patients with RA, a disease with irreversible effects." Abbott's global clinical trial database for HUMIRA is extensive and includes more than 14,000 RA patients representing more than 23,000 patient years of exposure to the medication. "Many patients with RA are not diagnosed early in the disease process and then may not be treated as quickly as they should, which can result in long-term, and sometimes irreversible, effects of the disease," said John Medich, Ph.D., divisional vice president, Clinical Development, Immunology, Abbott. "These long-term results on clinical response and radiographic inhibition provide additional data about the use of HUMIRA in long-standing RA and further demonstrate Abbott's continued commitment to improving the standards of care for patients with the disease." Clinical outcomes were assessed by Disease Activity Score 28 (DAS28), which is a composite index that includes variables such as the number of tender and swollen joints and either erythrocyte sedimentation rate or C-reactive protein (CRP), both of which are measures of inflammation. About DE019 DE019 was a Phase 3, randomized, controlled trial in which patients with long-standing RA and an inadequate response to MTX were randomized to one year of HUMIRA 40 mg every other week (ADA-40), HUMIRA 20 mg weekly (ADA-20) or placebo (PBO) injections; all received concomitant MTX. The 52-week controlled portion of the trial demonstrated the clinical and radiographic superiority of HUMIRA plus MTX over placebo plus MTX. Of the 619 patients initially randomized, a total of 202 patients (32.6 percent) continued on open-label HUMIRA plus MTX through year 10. This post-hoc analysis evaluated the radiographic data available at baseline and year 10 for patients who had data available for both time periods. Clinical and functional outcomes were assessed using the DAS28 and the Health Assessment Questionnaire Disability Index (HAQ-DI), which is a measure of a patient's physical function. Radiographic damage was assessed at baseline and years one, eight and 10 using the modified total Sharp score (mTSS), which records both bone and cartilage damage (joint erosion score and joint space narrowing score, respectively) on X-ray. After ten years in the study, patients had a mean DAS28 (CRP) of 2.6, mean HAQ-DI of 0.7 and a mean change in mTSS of 2.8. DAS28 (CRP) <2.6 was observed in more than half of patients who continued on HUMIRA for up to 10 years (59.6 percent). In the DE019 study, no new safety signals were identified following up to 10 years of HUMIRA exposure. About DE020 DE020 was a long-term, open-label, follow-up trial enrolling patients from four previous studies (including the ARMADA and STAR studies, as well as two smaller studies) in the early development program of HUMIRA. All four studies included patients with active RA and an inadequate response to MTX and/or other disease-modifying antirheumatic drugs (DMARDs). Patients could receive supplemental DMARD therapy at the investigator's discretion after inclusion in DE020. A total of 846 patients were enrolled in this follow-up study. Clinical and functional responses were assessed as the percentage of patients observed to achieve ACR criteria 20/50/70 responses, DAS28 <3.2 (a measure of low disease activity), DAS28 <2.6 and HAQ-DI <0.5 at year 10. ACR response criteria measure the percent improvement in tender and swollen joints, as well as patient and physician global assessment, pain, disability and inflammatory markers. A total of 286 patients (33.8 percent) completed 10 years of treatment with HUMIRA. DAS28 (CRP) <2.6 was observed in more than half of patients (57.2 percent) at year 10. A composite of both DAS28 (CRP) ≤3.2 and HAQ-DI <0.5 was observed in more than one-third (37.3 percent) of patients at year 10. At baseline, the patient population showed a mean DAS28 score of 5.7, indicating high disease activity. The mean DAS28 score at year 10 was 2.6. At baseline, the patient population had a mean HAQ-DI score of 1.4 (+/-0.6). At year 10, average HAQ-DI scores were observed to be 0.8 (+/-0.7). The following data were also observed at year 10: 78.6 percent of patients achieved at least 20 percent improvement in the ACR criteria (ACR20) 55.5 percent achieved at least 50 percent improvement in the ACR criteria (ACR50) 32.8 percent achieved at least 70 percent improvement in the ACR criteria (ACR70) 71.2 percent achieved a Disease Activity Score 28 [DAS28 (CRP) ≤3.2] 42.4 percent achieved a Health Assessment Questionnaire Disability Index (HAQ-DI) <0.5 No new safety signals were identified in this study following more than 10 years of HUMIRA exposure and rates of adverse events (AE) were consistent with the known safety profile of HUMIRA. About HUMIRA® (adalimumab) Uses HUMIRA (adalimumab) is a prescription medicine used alone, with methotrexate, or with certain other medicines to reduce the signs and symptoms of moderate to severe rheumatoid arthritis in adults. It may prevent future damage to bones and joints and may help with the ability to perform daily activities. Important Safety Information HUMIRA is a TNF blocker medicine that affects the immune system and can lower the ability to fight infections. Serious infections have happened in people taking HUMIRA. These serious infections include tuberculosis (TB) and infections caused by viruses, fungi, or bacteria that have spread throughout the body. Some people have died from these infections. People should be tested for TB before HUMIRA use and monitored for signs and symptoms of TB during therapy. People at risk of TB may be treated with medicine for TB. Treatment with HUMIRA should not be started in a person with an active infection, unless approved by a doctor. HUMIRA should be stopped if a person develops a serious infection. People should tell their doctor if they live in or have been to a region where certain fungal infections are common, have had TB, hepatitis B, are prone to infections, or have symptoms such as fever, fatigue, cough, or sores. For people taking TNF blockers, including HUMIRA, the chance of getting lymphoma or other cancers may increase. Some people have developed a rare type of cancer called hepatosplenic T-cell lymphoma. This type of cancer often results in death. If using TNF blockers including HUMIRA, the chance of getting two types of skin cancer (basal cell and squamous cell) may increase. These types are generally not life-threatening if treated. Other possible serious side effects with HUMIRA include hepatitis B infection in carriers of the virus, allergic reactions, nervous system problems, blood problems, certain immune reactions, including a lupus-like syndrome, liver problems, and new or worsening heart failure or psoriasis. The use of HUMIRA with anakinra or abatacept is not recommended. People using HUMIRA should not receive live vaccines. Common side effects of HUMIRA include injection site reactions (redness, rash, swelling, itching, or bruising), upper respiratory infections (including sinus infections), headaches, rash, and nausea. HUMIRA is given by injection under the skin. The benefits and risks of HUMIRA should be carefully considered before starting therapy. This is not a complete list of the safety information for HUMIRA. For additional important safety information, please click for the Full Prescribing Information and Medication Guide. About Abbott Abbott (NYSE: ABT) is a global, broad-based health care company devoted to the discovery, development, manufacture and marketing of pharmaceuticals and medical products, including nutritionals, devices and diagnostics. The company employs approximately 91,000 people and markets its products in more than 130 countries. |
|
New Drug Review (2008-12-15)
Adalimumab (Humira) for the Treatment of Rheumatoid Arthritis BETH WELCH, PharmD, Massachusetts College of Pharmacy and Health Sciences, Boston, Massachusetts American Family of Physician. 2008 Dec 15;78(12):1406-1408. Adalimumab (Humira) is a disease-modifying antirheumatic drug (DMARD) labeled for the adult treatment of moderately to severely active rheumatoid arthritis. It is a recombinant human immunoglobulin G1 monoclonal antibody that specifically binds and inhibits the inflammatory cytokine tumor necrosis factor (TNF)α.1 SAFETY There have been rare instances of serious infections, malignancies, and neurologic reactions with TNF antagonists, including adalimumab.1–4 Serious infections can be fatal and include sepsis, opportunistic infections, invasive fungal infections (e.g., histoplasmosis, coccidioidomycosis, blastomycosis, aspergillosis, candidiasis), and tuberculosis. Although rare, increased rates of lymphomas and malignancies (including, but not limited to, nonmelanoma skin, lung, or breast cancers) have been observed with the use of TNF antagonists.1,2 Adalimumab may rarely cause new-onset or exacerbations of neurologic disorders (e.g., paresthesia, tremor) or central nervous system-demyelinating diseases (e.g., optic neuritis, multiple sclerosis); caution should be exercised in patients with preexisting disease.1 The TNF antagonists, including adalimumab, have also been found to increase the risk of hepatitis B virus reactivation in chronic carriers.1 Cases of worsening heart failure have been reported with adalimumab.1 There is insufficient evidence to suggest differences among TNF antagonists with regard to incidence of serious adverse events.5 Adalimumab is U.S. Food and Drug Administration pregnancy category B.1 TOLERABILITY Adalimumab is well tolerated and the discontinuation rate because of adverse effects is similar to placebo (7 and 4 percent, respectively).1 Mild injection site reactions consisting of erythema and/or pain, itching, or swelling will occur in one in five patients and rarely necessitates drug discontinuation.1 Nonserious infection, including upper respiratory infection, bronchitis, and urinary tract infection, at a rate of one per patient-year will likely occur.1 However, most patients can continue therapy after infection resolution.1 Other adverse effects are rash and headache, which occur in less than 5 percent of patients.1 EFFECTIVENESS Adalimumab has been studied in adult patients with moderate to severe rheumatoid arthritis as an early single-agent intervention, as a substitute for traditional DMARDs in patients not receiving benefit, and in combination with traditional DMARDs. In patients with early aggressive rheumatoid arthritis, adalimumab produces similar results as methotrexate after one year of therapy.6 When used alone in early aggressive rheumatoid arthritis, adalimumab therapy can be expected to result in 23 percent of patients achieving remission and 41 percent of patients exhibiting clinical response after one year.6 However, when combined with methotrexate, the remission rates nearly double (43 percent) and response rates increase significantly (62 percent).6 After two years of combined therapy, benefit should still be evident, with close to one half of patients achieving remission, whereas only one fourth of patients on single-agent therapy will achieve remission.6 Adalimumab has also demonstrated benefit when substituted for or added to traditional DMARDs (e.g., methotrexate, leflunomide [Arava], sulfasalazine [Azulfidine], hydroxychloroquine [Plaquenil]) in patients who do not receive full benefit from their existing therapy. Over the short term (three months), 32 percent of patients who do not receive benefit with traditional DMARDs will experience a major clinical response when adalimumab is substituted.7 Clinical response is significantly better (41 to 43 percent response rate) if adalimumab is added to the existing therapy. This benefit appears to be most significant if the existing regimen contains methotrexate.7 There have been no direct comparisons between adalimumab and traditional DMARDs other than methotrexate. Likewise, TNF-alpha antagonists have not been compared directly, but adjusted indirect comparisons indicate similar response rates.8 PRICE Adalimumab costs approximately $1,662 per month, which is the same cost of the TNF-alpha inhibitor etanercept (Enbrel). The cost of methotrexate is approximately $13 to $85 per month. SIMPLICITY Adalimumab can be used as monotherapy or in combination with methotrexate or other traditional DMARDs. It should not be used in combination with anakinra (Kineret), and its use with other TNF antagonists is not recommended. The dose is 40 mg injected subcutaneously every other week. Adalimumab is dispensed in a prefilled, single-use pen or syringe for self-administration. It must be stored in the refrigerator. Adalimumab should not be initiated in patients with an active infection and should be discontinued if a serious infection occurs. Patients who experience weight loss, cough, fever, shortness of breath, or fatigue should discontinue the medication immediately and be evaluated for the presence of infection.4 Patients should be evaluated for latent tuberculosis infection before drug administration and monitored for signs and symptoms of active tuberculosis disease while on therapy. Those at risk of hepatitis B virus infection should be evaluated for carrier status before adalimumab initiation. Live vaccines should not be administered concurrently in patients receiving adalimumab.1 Bottom line Adalimumab used alone offers little benefit over the traditional first-line agent, methotrexate. However, it increases remission rates when combined with methotrexate in early aggressive rheumatoid arthritis and when combined with traditional DMARDs in those who have not derived benefit from existing regimens. Most patients experience few side effects; however, rare, serious adverse events can occur. Address correspondence to Beth Welch, PharmD, at [email protected]. Reprints are not available from the author. |
|
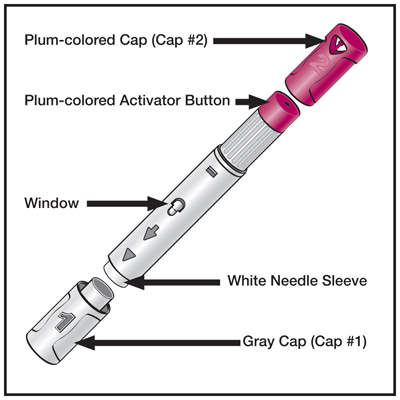
Adalimumab (Humira)
|
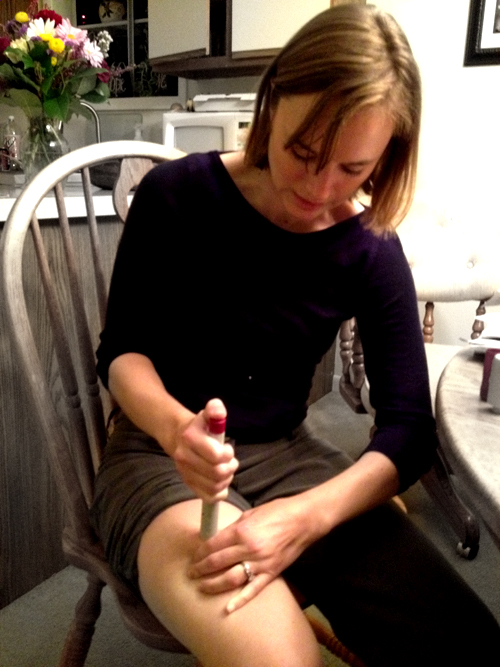
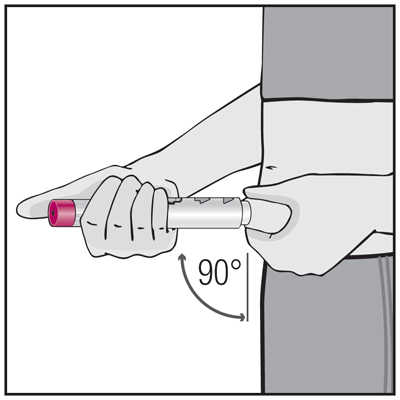
How to inject Humira PAINLESSLY!
|
|
Q: How long does Humira take to take affect?
(2009-3-14) My husband was diagnosed with RA about 9 months ago, and he is having a horrible time! He did ok on methotrexate for awhile, but then he had a terrible flare-up and his doctor prescribed Humira. So far he has had three shots, but the flares are continuing. Does anyone know how long it can take for Humira to work? He is SO depressed! It jumps around from hands to shoulders to feet to hips - its awful! Barb (2009-3-15) Hi, Is your husband on any other arthritic medication to work with the Humira? I ave been on Humira, Arava, Celebrex to help combat my arthritis. He should start feeling the effects of the Humira by the next 2 shots. I did by my 5th shot. Everyone is different. If he is still in a bad flare up, his doctor should have helped him to ease the flare up along with putting him on Humira. You and your Husband should call your doctor back to help ease his flare ups while on waiting for the Humira to work. Maybe Humira is not right for him. Talk to his doctor for other options that might help him now and continue to help him in the future, as for the depression, that comes with the arthritis, talk to his doctor about that also, he can help. Joanzie (2009-3-20) Thanks for the input! He is also on Methotrexate and he still has to take prednisone every day. He has had only 3 shots so far ( he takes one every 2 weeks), so I think we are going to give it a bit longer to see what happens. So far he has has lots of sporadic and widespread flare-ups, but the intensity seems to be diminishing somewhat. Maybe that's a sign that the Humira is starting to work - actually he is having a great "flare free" day today. We are still in the learning process here, so all the input and information I get on this website is so helpful. Thanks again. Sharon (2009-7-27) I too have just been diagnosed with RA it is horrible I couldnt dress myself or go to the washroom by myself ,I am a fairly active person and within 2 months I was almost bed ridden. I started Humira 2 weeks ago and athough I still have pain and stiffness it has taken the edge off, the first day I noticed a small changed but thought that it was in my head because I wanted relief so bad. I get my second injection on wed and am very hopeful that I will feel a bit better again. my pharmacist said it can take up to 6 months to feel the full effect . I could not have survived that long in the agony I was in . I too was depressed and still am a bit . But I think it will get better. hang in there. If no one has it they really dont know how much PAIN you are in... Joanzie (2009-7-27) Thanks for sharing. My husband never responded to Humira, but his doctor put him on Embrel and he has been mostly pain free for the last 7 weeks. And believe me, I understand the pain you are in; I may not have experienced it first hand (thank goodness), but I've seen my active, hard-working husband become debilitated by the pain. Even getting to the bathroom was a major ordeal for him during the worst of it. I hope everything works out for you, and please remember you are not alone. Schulz79 (2009-9-16) I turned 30 last March and was diagnosed the previous October. So I was only 29. I am male and my wife has had to watch all my painful symptoms as well. Just getting up from the chair to go to the restroom could be quite an ordeal. I have been on plaquenil and prednisone, now off the prednisone and my doctor just prescribed Humira. I had my first injection this past Saturday, and whether or not it is in my head, I believe it helped take some of the edge off the pain and stiffness. I have my fingers crossed for the next injection that it continues. RA has been a very painful disease and stairs were the first thing to go for me. I may not be able to ever conquer the stairs again, but walking normally will always satisfy me. Imagine how they look at you when you can walk into the BMV and get a handicap placard at age 30; people assume you are a faker. In reality, some days I can not walk very far at all. I understand all the pain your husband's must have, I too live it. |
|
JT (2009-10-28)
I am so happy to find this site! I was diagnosed with RA about a year ago but had been having symptons about a year prior to that. I had been tested for RA but came up negative. It was so bad living with pain and no meds besides ibuprofren to help the pain. They since have put me on Celebrex, Methotrexate, Predinisone, and now Humira. Yesterday was my first injection and it hurt a little more than I anticipated! Any suggestions on how to ease the sting? Shots don't bother me but may it was just because I did it myself. It's nice to hear that i'm not alone with RA. The pain, depression, and frustrations. There were mornings when I didn't think I could turn the door knob or start my car to take my son to school. And not being able to sleep because of the pain I think has been the hardest for me so far. I'm only 32 and it was so embarising for me in public when I could barley get up from a table or walking extremely slow because the bottoms of my feet hurt. I was like I would wake up and a different part of my body was hurting. It was crazy how the RA jumped around like it did. I'm finally coming out of my depression, able to sleep through the night, and can get around with very little pain. I feel like I have my life back again! Zenus442 (2009-11-19) take the humeria out of the fridge and leave it out for 25-30 minutes before you inject. I dont know why but it takes the sting out when you let it warm up a little bit. Also Ive found that injecting in my stomach is easier then when I use to inject in the leg. Frankie (2009-11-23) I leave the Humira pen out for 30 minutes before I give myself the shot. Make sure you clean the site properly with an alcohol wipe and cover the area with a bandaid. I inject my stomach area away from the belly button. It does hurt a little, but nothing like the pain from the RA. My first two shots gave me a nasty rash which took a couple of weeks to clear up. Since then I seem to be fine. I once injected my thigh when I was on Enbrel, but it hurt really badly. So, I am going to stay with my stomach. Lots of luck to you and I hope the treatment works. Pam (2010-5-5) I know I am a little late responding, but just found this website. I let the shot warm up for about 45 minutes so it is room temp. Then before I give the shot I slap the leg a few times. This really made a difference for me. Mrome (2012-2-11) I am 47 year old female and I was diagnosed back in year 2000 but never got treatment because I just dealth with the pain but this year and half I have become somewhat incapcitated. I walk with a limp, stairs kill me. Opening a bottle or a jar is excruitiating. I started with metrotrexate pills but became ill with vomiting and bleeding through my nose. The next step was avara. I had terrible side effects, my nervous systems went crazy, blurred vision, swollen eyes, loss control of my nerves. Then I started metrotrexate injections .5mg weekly did not help, .8mg slight relief but my levels are still high. I started my first Humira injection this Saturday and I was totally surprised by the kick it had. I am used to the burning sensation from the metrotrexate, I too am desperate for it to work because I can't sleep for every bone in my body wreaks of pain. So you can't sleep so you are so tired and the depression and anxiety kicks in. I pray for all of us to find some help and relief. It saddens me that unfortunately it takes so much trial and error to find a medication that works for us. Char (2013-11-7) I WAS DIAGNOSED ABOUT 2 YRS AGO. ON METHOTREXATE (HORRIBLE STUFF, IT IS AN OLD CANCER DRUG). I HAVE HAD ABOUT 6 SHOTS OF HUMIRA AND I DO THINK IT DOES HELP. NOW IT HAS STARTED IN MY FEET AND KNEES. I GET UP ABOUT 3:00 A.M. JUST TO LOOSEN UP TO GO TO WORK. THIS IS THE LAST THING I THOUGHT I WOULD EVER GET! ANYWAY, WE NEED MORE SUPPORT GROUPS. I TOTALLY AGREE WITH THE TIREDNESS, DEPRESSION ETC. MY HUSBAND HAS REALLY PUT UP WITH ALOT. I AM VERY LUCKY. I FORGOT WHAT IS FEELS LIKE TO FEEL GOOD, MAYBE SOME DAY. HANG IN THERE, WE ALL KNOW WHAT YOU'RE DEALING WITH. Carla (2012-10-15) I have been on Humira for 3 weeks now and it has been a miracle for me!! My neck, shoulder and hip were unbearable. Now I am feeling like a million bucks!! I too had relief after my first dose. Hard to believe but it is wonderful. Good luck!! Hope??? (2012-9-16) Hi Tina, Yes, you're so right. It is very hard on the carer. My husband has been great! He was struck by lightning 3 years ago; the day before my neck & back reconstruction. I recuuperated & then got R.A. and now Fibromyalgia. In so much pain! Started with Orencia then after a year switched to Embrel & now my 3rd year, Humira. Each med becomes ineffective after a year. Hopefully Hurmira will be the one to put me in remission. I watch what I eat, but with lack of activity & med's, I can't loose weight! I'm trying to see how long the Hurira takes to work. The first dose didn't cause any improvement. Am so stiff, swollen, and sore. The Enbrel worked right away! Best of luck to everyone! This is a terrible disease that causes one to loose hope. That's something I've never had to confront before. I usually am very positive. God bless us and keep us healthier then today!!! Charm1 (2013-1-24) Hi start the Humira next week....my knee's are inflammed so bad that I can barely walk....I took metrothrexate(sp) and it made my hair fall out and my immune system go down...then I just planned was to scared to go back to the doctor, and tried naturalpath wish was a mistake, it did not work...I went to a female Rhumatologist and she was very informative and told me not to be scared of the humira...there is so much side effects in anything over the counter...like Aleve, Iburoprofin etc....she said the side effects in the Humira are very rare, but they have to list them no matter what...so I am sucking it up and starting next week...I hope you can get back to me on how you are doing on it..I have trips coming up this summer and want to get well...I can barely walk and half the time I am in a wheel chair which sucks..I have always been active, and want to be back to that...take care and I hope you are doing well Lind (2013-6-6) Just like everyone else, Im thrilled to find this outlet for those of us with RA. I was diagnosed in 2009, although Ive had symptoms for longer. Im currently taking MTX, plaquenil, Tramadol, and just started Humira last month. So far, no relief. I also have Celiac Disease and Sjogrens. I too, am losing my hair. Has anyone filed for disability? Has anyone been approved? Im 46 and am an RN. I went from working 60 hrs/wk to 5hrs/mo! I often get confused and I lose my balance, having to use a cane. I had to go off all my meds for 2 mos due to adverse reactions to Arava. It was amazing how sharp my mind was, but the pain was unbearable. I think the MTX is the culprit. Like everyone else, Im hoping the Humira will kick in soon. Thanks for listening (reading?) to my rambling. :0) Linda Karisaficek (2009-9-23) I noticed a difference instantly. not a significant difference...but I am 24 and have had RA since 4 years old and let me tell you i can actually clean my house and care for my 2 year old so that is how I know there is a difference. I live in rainy washington state so the weather kills me...but there have been several rainy days and i can function. I am also taking methotrexate, tramadol and percocet but i don't take pain med half the time because I cannot function with my child while I am on them. it is nice to read that others are feeling my pain. I was an aircraft mechanic for the military making extremely good money and lost it all a year ago because my RA has just become crippling. it is so hard to support a child with no income. also, I took enbrel and got stomach inflammation and extreme diahhrea from it. and a week ago I was also diagnosed with fibromyalgia...if I can keep going you can. yes, there are days i keep giong but i am relying on this little ray of hope that my doctor can find the right regimen of meds that will work for me. if I didn't have that hope and him telling me he is positive he can help me, and getting up each day with my child to live for, he gives me purpose. I think I would have killed myself had I not had such a magnificent child to live for. |
|
Pamela (2010-8-21)
I have just signed on here. I'm not even sure i've repleid properly. So here i am at 53 years old and i've had this RA since i was 30. It's a very long story so i won't go into that right now. But i'm on Methotrexate for the pas 15 years and had a small dose of cortisone (5 mg) a day, plus anti-inflammatory and pain killers. I went on Embrel a few years ago but it didn't work. Then Humira a year and a half ago. It worked within 3 days. I felt a new woman. But then i got a cold which turned into Bronchitis and i ended up going to hospital by ambulance with breathing stress. So i stopped the Humira. Since christmas i am suffering terrible with my left knee. So thought i should go back on Humira. I have had my 5th shot yesterday and strangely it isn't working at all. I live in France and it is almost 3 am and that's why i'm here because i'm suffering like mad. it's preventing me from sleeping. Doesn anyone know why Humira worked for me a year ago and why doesn't it work now. i'm so very peeved with this. I had accepted this illness and tried to get on with life, with 5 kids and a great husband. But now i am getting really depressed like in the beginning because i'm feeling that my life should be starting over with the kids grown and independant and it's all going wrong. I know i'm ranting but i'm so desperate. i've really appreciated what i've read here. I think there is a 3rd anti TNF (another type of Humira, Enbrel). I wonder if that would work. There doesn't seem to be a light at the end of this tunnel. Thanks for listening to my ramblings. By the way i live in France but i'm English. T607 (2011-7-15) I'm so sorry to hear that. Unfortunately, Humira does not work for everyone. It didn't work for me. After two months of feeling no relief, I am right back on prednisone and methotrexate where I started. Although, I still feel a lot of pain, without them it would be worse. I was very angry. I hear people talk about humira, celebrex, and enbrel all the time stating how well they work. Well, none of them worked for me. My doctor thinks because I've been on prednisone since 1996 that my body is so used to it nothing else has a shot in hell. I think he has lost it. There has got to be something out there for me. Thank God, my husband is very supportive. I've been living with this since I was 25 years old. The meds give you mood swings, make you angry, tired, depressed, you name it, I've been there. Hang in there and God bless! Your hubby is lucky to have you. Jean (2011-7-18) Hi- I just found this site looking for information about humira. My 13 year old daughter was diagnosed with JRA a year and a half ago and we have been working hard with her doctors to find what works. My daughter was on enbrel for 6 months with good results along with methotrxate but then it stopped working as well so her doctor put her on Humira. She' had 2 doses with no relief. She is so much worse, having daily flares. She can't do the things she was able to do on the enbrel. I am trying to find out how much longer it'll be until it starts being effective. She frustrated and angry. She's been taking prednisone for 2 weeks to help carry her through until the humira kicks in, but we don't want her on it for long. She's having lots of flares, pain, fatigue even on prednisone. I don't know how long we should make her wait to see if humira will work and how can we tell if she's taking prednisone. She's being robbed of her youth as we struggle to find solutions that will help her. Janak (2012-12-3) I am 37 and was diagnosed in July after symptoms started presenting themselves at the end of January. I was a runner, super healthy and in great shape. I guess taking care of yourself doesn't count for much these days. :( I was on MTX 20mg and 5mg Pred, but it wasn't cutting it - though I did feel a ton better. I started on Humira a little over a month ago and had my 3rd shot 3 days ago. I felt huge results the morning after my first shot. My pain simply almost vanished. My hands didn't hurt or anything. I couldn't believe it. I tried to wean off the prednisone a couple weeks latter, but could feel the pain coming back so I went back up to 5 mg 1x/day. Right now I can feel it in my elbows a bit, but my hands are looking great and I am back to running again. I am super out of shape now, but have been able to run with little walking a little over 3 miles 3x a week with now pain. Hopefully it just keeps working so that I can get off the prednisone and wean down on the MTX. |