現在位置 : 藥物 > 消化性潰瘍藥 - Esomerprazole (Nexium)
|
Esomerprazole (埃索美拉唑镁)
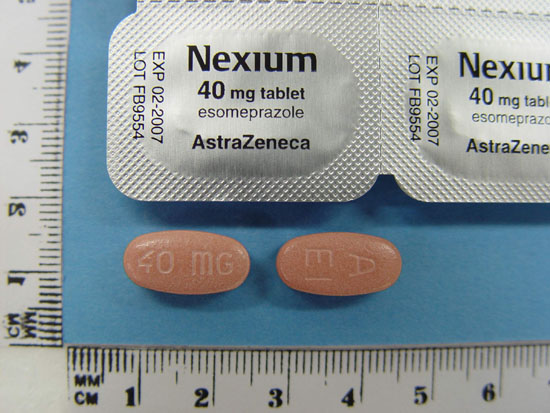
商品名 : 台灣健保藥品 - Nexium 耐適恩錠 美國- Nexium 加拿大 - Apo-Esomeprazole; Nexium Nexium (阿根廷, 奧地利,澳大利亞, 玻利維亞, 巴西,瑞士, 智利, 德國, 丹麥, 西班牙, 芬蘭, 希臘, 香港, 印尼, 愛爾蘭, 以色列,義大利, 馬來西亞, 荷蘭, 挪威, 紐西蘭, 秘魯, 菲律賓, 波蘭, 葡萄牙, 俄羅斯, 瑞典, 新加坡, 泰國, 土耳其, 委內瑞拉, 南非) 藥物作用 Esomeprazole為一種稱為「氫離子幫浦阻斷劑」類的藥物。此類藥物具有顯著及長效﹑降低「胃酸分泌」的作用。它可以用來治療胃潰瘍或反流性食道炎及其它胃酸過多引起的病症。胃潰瘍的發生,往往是由於胃細胞分泌過量的胃酸,導致胃受到過度的刺激,而造成胃壁潰爛的現象。反流性食道炎的造成是由於胃酸回流入食道,造成食道的腐蝕潰爛。此藥的作用,就是能直接減少胃酸的產生,降低胃或食道的刺激,使得潰瘍部份漸漸地得到休息康復。此藥也可與其它抗生素一起服用﹑治療由H. pylori 細菌感染﹑所造成的胃潰瘍。 用法 此藥通常是一天服用一次。由於食物會降低此藥的吸收,因此應在飯前一小時服用。服藥期間,最好養成每天在固定時候服藥的習慣,以減少忘記。由於此藥為一緩慢釋放型膠囊,因此應該整粒吞服,不可咀嚼服用。如果覺得膠囊過大,則可將膠囊打開,將裡面的小顆粒擺在柔軟的食物上或飲料中使用,切忌在嘴中咀嚼,以免膠囊裡面的小顆粒破碎,藥物中的有效成分受到胃酸的破壞﹑而降低藥效。 注意事項 禁忌症:對 Esomeprazole 或其它「氫離子幫浦阻斷劑」類藥物如﹕Omeprazole, Lansoprazole, Pantoprazole過敏者不該使用此藥。 如果懷孕,對藥物過敏,或者有肝臟疾病等,醫師需要針對這些情況謹慎用藥或調整劑量﹑因此在使用前,應該事先通知醫師。 在服用此藥後的幾天,胃痛的症狀不見得能夠立即得到改善。但是,不可因為一時覺得沒有藥效而停止服用。除了醫師特別禁止外,可使用制酸劑以減輕疼痛。通常在服用此藥一兩個星期後,胃潰瘍的症狀應該會得到相當程度的改善,但是千萬不可因為一時覺得已經痊癒,或者覺得胃不痛就停止服用,應該依照醫師的指示,完成整個服藥的過程。對於某些程度的潰瘍,也許需要6至8個星期才可以痊癒。 市面上許多治療頭痛﹑關節痛以及肌肉痛等止痛藥物,會對胃產生極大的刺激,也許會使胃潰瘍更為惡化。因此在使用此類藥物前,應該詢問醫師或藥師,何種藥物對胃最不會造成傷害。 服用此藥期間,最好能少量多餐﹑避免食用辛辣酸性的食物﹑降低使用煙酒的次數﹑穿著較微寬鬆的衣物﹑避免飯後立即躺下等等。都可間接的減緩胃酸對胃部或食道的刺激﹑增進藥物痊癒的時間。 胃腸內酸鹼性的改變﹑有可能會直接或間接的影響其它藥物在體內的吸收﹑由於此藥降低胃酸的分泌﹑藥物在體內的吸收因此可能受到改變。譬如它能增加Digoxin的藥效﹑降低Ketoconazole的作用。為了避免突然增強或降低其它藥物的藥效,服用此藥前,最好能事先告訴醫師有服用那些藥物。 副作用 Esomeprazole最常見的副作用為: 腹痛(1%至3.8%)﹑便秘(1%以上),腹瀉(1%至9.2%),脹氣(1%或以上)﹑噁心(1%至2%)﹑口乾(1 %或以上)﹑頭痛(1.9%至8.1%),嗜睡(1.9%)等。其它可能副作用包括:便秘﹑口乾﹑胃腸不適﹑嘔吐﹑頭暈等。這些副作用,通常在服用藥物一陣子後,應該會漸漸地消失。不過,如果這些副作用達到困擾你的程度,或者經過一段時間後,還不能完全消除,就應該通知醫師。 此藥造成嚴重副作用及過敏反應的機率較低,但如果您有皮膚發紅﹑發燒﹑臉部腫大﹑呼吸困難等藥物過敏的現象﹑喉嚨痛就應該盡快通知醫師。 懷孕及哺乳 美國藥管局(FDA)將此藥歸類為懷孕類別B級:B級表示動物研究結果無法證實藥物會對胎兒造成傷害﹑但沒有足夠的對照孕婦實驗證明其安全或危險性﹑使用此藥一般而言是安全的。或者是動物的研究已經顯示出藥物會對於動物胎兒造成某種程度的副作用﹑但人體對照組研究結果無法在懷孕任何一期中證實藥物會對胎兒造成傷害。 目前為止﹑動物實驗顯示在正常劑量下﹑此藥造成胎兒生長缺陷的機會不大,然而﹑動物實驗結果與人體反應並不一定完全相同﹑藥物使用於孕婦的資料有限。因此懷孕時,仍然需要通知醫師,他會衡量狀況,決定是否應該服藥。 Micromedex公司哺乳評級結論﹕ 對嬰兒的風險性還無法排除。 (由於現有資料不足或專家結論不同情況下﹑無法充分證明哺乳期間使用藥物是否會增加嬰兒的風險。婦女若考慮使用母奶哺乳嬰兒﹑應該權衡藥物的優點或藥物對嬰兒潛在的風險性。) 目前為止,尚不知此藥是否會經由母乳到達嬰兒體內,但是為了避免對新生兒產生影響,餵奶的母親在使用此藥時,應該停止餵食母乳,而改用其它的乳製品取代。 忘記用藥 如果忘記服藥,應該在記得時,立即服用,但是,如果距離下次服藥的時間太近,就應該捨棄所遺忘的藥物,恢復到下次正常服藥的時間,千萬不可一次服用雙倍的劑量。 |
|
藥理作用
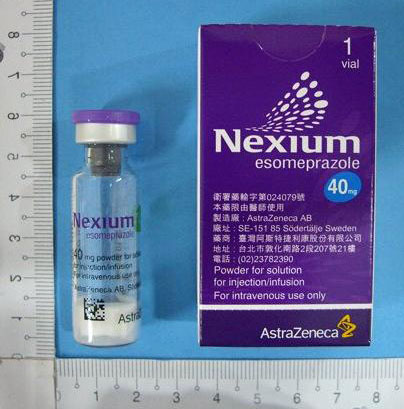
Esomeprazole為omeprazole之S型光學異構物,經由作用於專一目標之作用機轉,降低胃酸分泌。其為胃壁細胞內酸幫浦之專一性抑制劑。Omeprazole的R及S型光學異構物二者有相似的藥效活性。 作用部位及機轉: Esomeprazole為一弱鹼,會在胃壁細胞內分泌小管之高度酸性環境下集中,並轉化成活性型,其可抑制酸幫浦:H+-K+-ATP酵素,因此對基礎胃酸分泌及因刺激產生之胃酸分泌,均有抑制效果。 適應症 耐適恩注射劑用於不適合使用口服治療時之替代治療: -胃食道逆流性疾病,於食道炎及/或有嚴重逆流症狀病患 -NSAID治療相關之胃潰瘍的治療於治療性內視鏡處置急性出血性胃潰瘍或十二指腸潰瘍之後預防再出血。 用法用量 不能經口投藥時的抗胃酸分泌治療: 對於不能接受口服藥的患者,可注射20-40 mg每天一次加以治療。對於逆流性食道炎的患者,應該用40 mg每天一次加以治療。針對逆流性疾病之症狀治療則以20 mg每天一次加以治療。 NSAID治療相關之胃潰瘍的治療:一般劑量為20 mg每天1次。通常靜脈注射治療期很短,應該儘速改為口服治療。 預防胃潰瘍與十二指腸潰瘍再出血用治療性內視鏡處置急性出血性胃潰瘍或十二指腸潰瘍之後,應以快速靜脈輸注的方式給予80 mg,輸注時間30 分鐘,隨後以8 mg/小時的速率連續靜脈點滴輸注3 天(72 小時)。非經腸治療期之後應接續口服胃酸抑制治療。 藥動力學 分佈: 健康受試者在穩定狀態下的擬似分佈體積大約為0.22 L/Kg,esomeprazole的血漿蛋白結合率為97%。 代謝及排除: Esomeprazole完全由細胞色素P450系統(CYP)代謝。Esomeprazole主要由多形性之CYP2C19代謝,負責形成esomeprazole之羥基代謝物及去甲基代謝物。 Esomeprazole之主要代謝物對胃酸分泌並無影響。esomeprazole口服劑量中,有80%以其代謝物型式由尿中排除,其餘則由糞便排除。只有低於1%之原型藥可在尿液中發現。 副作用 頭痛、腹痛、便祕、腹瀉、腹脹、噁心/嘔吐。 交互作用 Esomeprazole對其他藥物之藥動學影響: 吸收會受胃液pH值影響的藥品以esomeprazole治療時,因胃中酸度會降低,故如果藥物吸收之機轉會受胃酸強度影響時,可能會使藥物之吸收增加或減少。與使用其他胃酸分泌抑制劑或制酸劑相同,在esomeprazole治療期間,ketoconazole及itraconazole的吸收會減少。 其他藥物對esomeprazole藥動學之影響 Esomeprazole由CYP2C19及CYP3A4所代謝。Esomeprazole與CYP3A4抑制劑clarithromycin (500 mg b.i.d.) 併服時,會使esomeprazole之暴露量(AUC)增加一倍;Esomeprazole與同時是CYP2C19抑制劑又是CYP3A4抑制劑的藥品併服時,可能會使esomeprazole之暴露量增加一倍以上。CYP2C19及CYP3A4抑制劑voriconazole會使omeprazole AUCt 增加280%。在以上二種情況下通常無須調整esomeprazole之劑量。然而,對於肝功能嚴重不良的患者以及有長期治療需求時,應該考慮調整劑量。 禁忌 1. 已知對其活性成分esomeprazole、其他benzimidazole 取代物或藥品中賦形劑有過敏反應之患者。 2. Esomeprazole 像其他PPIs 一樣,不可與atazanavir 併用。 給付規定 7.1消化性潰瘍用藥: 1.藥品種類: (1)制酸懸浮劑: 各廠牌瓶裝、袋裝制酸懸浮劑及袋裝顆粒制酸劑。 (2)乙型組織胺受體阻斷劑: 各廠牌乙型組織胺受體阻斷劑之口服製劑與針劑。 (3)氫離子幫浦阻斷劑: 各廠牌氫離子幫浦阻斷劑。 (4)細胞保護劑:如gefarnate、cetraxate、carbenoxolone等。 (5)其他消化性潰瘍用藥: dibismuth trioxide, sucralfate, pirenzepine HCl, Gaspin, Caved-S, misoprostol, proglumide及其他未列入之同類藥品,價格與其相當者比照辦理。 2.使用規定: (1) 使用於治療活動性(active)或癒合中(healing)之消化性潰瘍及逆流性食道炎。(92/10/1) (2) 瘢痕期(scar stage)之消化性潰瘍復發預防,其劑量依照醫理減量使用。 (3) 消化性潰瘍及逆流性食道炎符合洛杉磯食道炎分級表(The Los Angeles Classification of Esophagitis※備註)Grade A或Grade B者,欲使用消化性潰瘍用藥,其使用期間以四個月為限,申報費用時需檢附四個月內有效之上消化道內視鏡檢查或上消化道X光攝影報告,其針劑限使用於消化道出血不能口服之病人急性期替代療法。(92/10/1) (4)經上消化道內視鏡檢查,診斷為重度逆流性食道炎,且符合洛杉磯食道炎分級表(The Los Angeles Classification of Esophagitis※備註)Grade C或Grade D者,得經消化系專科醫師之確認後可長期使用消化性潰瘍用藥一年。另外,下列病患得比照辦理:(92/10/1) Ⅰ胃切除手術縫接處產生之潰瘍。 Ⅱ經消化系專科醫師重覆多次(三次以上)上消化道內視鏡檢查確認屬難治癒性之潰瘍。經診斷確定為Zollinger-Ellision症候群之病患,得長期使用氫離子幫浦阻斷劑而不受一年之限制。 (5)需使用NSAIDs而曾經上消化道內視鏡或X光攝影證實有過潰瘍,得於使用NSAIDs期間內,經消化系專科醫師之確認後可使用消化性潰瘍用藥。(92/10/1) (6)對於症狀擬似逆流性食道炎之患者,但其上消化道內視鏡檢查無異常,若欲使用消化性潰瘍用藥,則需檢附其他相關檢查(如24小時pH監測)的結果。(92/10/1) (7)消化性潰瘍穿孔病人經手術證實者,且所施手術僅為單純縫合,未作胃酸抑制相關手術者,可檢附手術記錄或病理檢驗報告,申請使用消化性潰瘍用藥,但以四個月內為限,如需繼續使用,仍請檢附胃鏡檢查或上腸胃道X光檢查四個月內有效報告影本。(92/10/1) (8)嚴重外傷、大手術、腦手術、嚴重燙傷、休克、嚴重胰臟炎及急性腦中風者為預防壓力性潰瘍,得使用消化性潰瘍藥品。此類藥物之針劑限使用於不能口服之前述病患短期替代療法。 (9)消化性潰瘍病患得進行初次幽門螺旋桿菌消除治療,使用時需檢附上消化道內視鏡檢查或上消化道X光攝影報告並註明初次治療。(92/10/1) (10)幽門螺旋桿菌之消除治療療程以二週為原則,特殊病例需延長治療或再次治療,需檢附相關檢驗報告說明理由。 (11)下列病患若因長期服用NSAIDs而需使用前列腺素劑(如misoprostol),得免附胃鏡報告,惟需事前報准後使用:(99/7/1) Ⅰ紅斑性狼瘡。 Ⅱ五十歲以上罹患類風濕性關節炎或僵直性脊椎炎之病患。 備註: 1.醫療院所使用單價新台幣四元(含)以下之消化性潰瘍用藥時,得由醫師視病情決定是否需要上消化道內視鏡檢查。(92/10/1) 注意事項 當任何警示性症狀出現時(例如體重無故地顯著減輕、反覆嘔吐、吞嚥困難、吐血或黑糞),以及當懷疑或已存在胃潰瘍時,應先排除惡性腫瘤的可能性,因以Nexium 治療會減輕其症狀,而延誤其診斷。 過量處理 至目前為止,尚無故意服用本藥過量之經驗。與280 mg之口服劑量有關的症狀為胃腸症狀與無力。單一劑量口服esomeprazole 80 mg及靜脈注射308 mg 靜脈注射24小時並未發生任何狀況。目前並無特定之拮抗劑。Esomeprazole與血漿蛋白廣泛地結合,因此以血液透析亦不易迅速排除。如果發生服藥過量,應採取症狀療法及一般支持性療法。 藥品保存方式 為了避光,請存於原包裝內。若拆除外盒後,小藥瓶暴露在一般室內光線下最多可儲存24小時。儲存勿超過25℃。 |
|
Esomeprazole
Esomeprazole is a proton pump inhibitor (brand name Nexium, Essocam) which reduces acid secretion through inhibition of the H+ / K+ ATPase in gastric parietal cells. By inhibiting the functioning of this transporter, the drug prevents formation of gastric acid. It is used in the treatment of dyspepsia, peptic ulcer disease, gastroesophageal reflux disease, and Zollinger-Ellison syndrome. Esomeprazole is the S-enantiomer of omeprazole (brand name Prilosec/Losec). Generic versions are available in several countries of Europe and in emerging markets such as Pakistan, India, Peru, Ecuador, Caribbean Islands, Nigeria, Africa, Sri Lanka, Cambodia, and Myanmar under the brand name Raciper. In Bangladesh, esomeprazole is sold under the brand name Opton by Beximco Pharma, Neptor by Sandoz Bangladesh. The primary uses of esomeprazole are gastroesophageal reflux disease, treatment and maintenance of erosive esophagitis, treatment of duodenal ulcers caused by H. pylori, prevention of gastric ulcers in those on chronic NSAID therapy, and treatment of gastrointestinal ulcers associated with Crohn's disease. |
|
Medical use
Gastroesophageal reflux disease Gastroesophageal reflux disease (GERD) is a condition in which the digestive acid in the stomach comes in contact with the esophagus. The irritation caused by this disorder is known as heartburn. Long-term contact between gastric acids and the esophagus can cause permanent damage to the esophagus. Esomeprazole reduces the production of digestive acids, thus minimizing their effect on the esophagus. Duodenal ulcers Esomeprazole is combined with the antibiotics clarithromycin and amoxicillin (or metronidazole in penicillin-hypersensitive patients) in the 7-14 day eradication triple therapy for Helicobacter pylori. Infection by H. pylori is the causative factor in the majority of peptic and duodenal ulcers. Evidence of efficacy AstraZeneca claims that esomeprazole provides improved efficacy, in terms of stomach acid control, over the R enantiomer of omeprazole. Many noted health professionals, including Dr. Otis Brawley (author of "How We Do Harm: A Doctor Breaks Ranks About Being Sick In America", and currently (as of August 2012) both chief medical officer and executive vice president of the American Cancer Society), have expressed the view that this improvement in efficacy is due to the dose of esomeprazole recommended for therapy rather than any inherent superiority of esomeprazole. In his 2003 address to the American Medical Association, Thomas Sully, then director of the Center for Medicare and Medicaid Services told his audience, "You should be embarrassed if you prescribe Nexium because it increases costs with no medical benefits…. The fact is Nexium is Prilosec …it is the same drug. It is a mirror compound." An alternative rationale suggested for the use of esomeprazole was the reduction in interindividual variability in efficacy. However, the clinical advantage of this hypothesis has not thoroughly been tested in large-scale trials. Given the large difference in cost between all other proton pump inhibitors and that of omeprazole, many physicians recommend a trial of over-the-counter products before beginning more extensive therapies and testing. Although the (S)-isomer is more potent in humans, the (R)-isomer is more potent in the testing of rats, while the enantiomers are equipotent in dogs. Adverse effects Common side effects include headache, diarrhea, nausea, flatulence, decreased appetite, constipation, dry mouth, and abdominal pain. More severe side effects are severe allergic reactions, chest pain, dark urine, fast heartbeat, fever, paresthesia, persistent sore throat, severe stomach pain, unusual bruising or bleeding, unusual tiredness, and yellowing of the eyes or skin. Proton pump inhibitors may be associated with a greater risk of hip fractures and Clostridium difficile-associated diarrhea. By suppressing acid-mediated breakdown of proteins, antacid preparations such as esomeprazole lead to an elevated risk of developing food and drug allergies. This happens due to undigested proteins then passing into the gastrointestinal tract where sensitisation occurs. It is unclear whether this risk occurs with only long-term use or with short-term use, as well. Patients are frequently administered the drugs in intensive care as a protective measure against ulcers, but this use is also associated with a 30% increase in occurrence of pneumonia. Interactions Esomeprazole is a competitive inhibitor of the enzymes CYP2C19 and CYP2C9, and may therefore interact with drugs that depend on them for metabolism, such as diazepam and warfarin; the concentrations of these drugs may increase if they are used concomitantly with esomeprazole. Conversely, clopidogrel (Plavix) is an inactive prodrug that partially depends on CYP2C19 for conversion to its active form; inhibition of CYP2C19 blocks the activation of clopidogrel, thus reducing its effects. Drugs that depend on stomach pH for absorption may interact with omeprazole; drugs that depend on an acidic environment (such as ketoconazole or atazanavir) will be poorly absorbed, whereas drugs that are broken down in acidic environments (such as erythromycin) will be absorbed to a greater extent than normal. Pharmacokinetics Single 20– to 40-mg oral doses generally give rise to peak plasma esomeprazole concentrations of 0.5-1.0 mg/l within 1–4 hours, but after several days of once-daily administration, these levels may increase by about 50%. A 30-minute intravenous infusion of a similar dose usually produces peak plasma levels on the order of 1–3 mg/l. The drug is rapidly cleared from the body, largely by urinary excretion of pharmacologically inactive metabolites such as 5-hydroxymethylesomeprazole and 5-carboxyesomeprazole. Esomeprazole and its metabolites are analytically indistinguishable from omeprazole and the corresponding omeprazole metabolites unless chiral techniques are employed. Dosage forms Esomeprazole is available as delayed-release capsules in the United States or as delayed-release tablets in Australia, the United Kingdom, and Canada (containing esomeprazole magnesium) in strengths of 20 and 40 mg, and as esomeprazole sodium for intravenous injection/infusion. Oral esomeprazole preparations are enteric-coated, due to the rapid degradation of the drug in the acidic conditions of the stomach. This is achieved by formulating capsules using the multiple-unit pellet system. Multiple-unit pellet system Esomeprazole capsules are formulated as a "multiple-unit pellet system" (MUPS). Essentially, the capsule consists of extremely small enteric-coated granules (pellets) of the esomeprazole formulation inside an outer shell. When the capsule is immersed in an aqueous solution, as happens when the capsule reaches the stomach, water enters the capsule by osmosis. The contents swell from water absorption, causing the shell to burst, and releasing the enteric-coated granules. For most patients, the multiple-unit pellet system is of no advantage over conventional enteric-coated preparations.[citation needed] Patients for whom the formulation is of benefit include those requiring nasogastric tube feeding and those with difficulty swallowing (dysphagia). Production and manufacture The granules are manufactured in a fluid bed system with small sugar spheres as the starting material. The sugar spheres are sequentially spray-coated with a suspension containing esomeprazole, a protective layer to prevent degradation of the drug in manufacturing, an enteric coating, and an outer layer to reduce granule aggregation. The granules are mixed with other inactive excipients and compressed into tablets. Finally, the tablets are film-coated to improve the stability and appearance of the preparation. Economics Between the launch of esomeprazole in 2001 and 2005, the drug has netted AstraZeneca about $14.4 billion |